Choose your Article Focus | NGS | Molecular & Serology
4 Best Practices for QC in a Clinical Testing Lab to Ensure Accurate Results
Category: Molecular & Serology
Posted by
Eric Morreale, PhD on Feb 18, 2020 12:00:00 AM
What is a clinical laboratory director’s worst nightmare? That’s an easy one. The thought that, somehow, an inaccurate testing result has escaped their lab and convinced an unsuspecting clinician to make a poor patient care decision. So much of modern medical care is grounded in treatment decisions based on diagnostic testing results. According to one review of the data, 98 percent of in-patient populations get lab tests. False negatives can delay life-saving and life-improving patient care. False positives can cause patients to undergo unnecessary treatment, which can result in needless psychological, physical, and financial distress. No wonder lab directors are troubled by the possibility, no matter how small, of reporting inaccurate results - critical patient care decisions hang in the balance. (Not to mention the professional reputations of lab directors, the reputations of their labs and hospitals, and the trust of patients and clinicians.) To prevent errors in testing and reporting, quality control is a must. You need to be confident every time you report a result that your assays are working the way they are intended. Here are four tips for optimizing quality control to ensure accurate results in your clinical lab:
0 Comments Click here to read/write comments
How to Comply With CLIA and Pass PT With Ease
Posted by
Eric Morreale, PhD on Feb 11, 2020 12:00:00 AM
Proficiency testing under CLIA is no joke. Failed tests lead to costly downtime for equipment and personnel as labs troubleshoot to find the source of an error. In the case of proficiency testing (PT), failures lead to time invested in corrective-action measures, and in the worst case, cease-testing directives from regulatory authorities. A failed CLIA proficiency test — multiple failures, especially — is the ultimate black mark not only on the career of a lab director but on the reputation of a clinical lab. The key to CLIA confidence is the same simple approach you took to ace your exams back in college: preparation. If you run your assays through their paces on a regular basis, and you use high-quality third-party controls to root out any weak points in your testing protocol and train your lab staff, CLIA proficiency testing will be a breeze.
0 Comments Click here to read/write comments
For Clinical Labs: Avoid False-Positives and Negatives by Being a Control Freak
Category: M&S
Posted by
Eric Morreale, PhD on Nov 26, 2019 12:00:00 AM
Do you consider yourself a control freak? And by control, we mean quality control - the procedures and materials implemented to ensure test accuracy and precision. Most importantly, having the proper controls in place provides confidence in the accuracy of your tests and the reported results, reducing the risk of generating false-positives or false-negatives. A strong quality program has the added benefits of reducing both the amount of valuable staff time expended on troubleshooting, as well as costly instrument and assay downtime.
0 Comments Click here to read/write comments
How Third-Party Controls Can Help Keep Your Clinical Lab Within Budget (Without Sacrificing Quality)
Category: M&S
Posted by
Eric Morreale, PhD on Nov 19, 2019 12:00:00 AM
As a clinical lab director, you are driven by two forces which, at first glance, may seem in opposition to each other. The first is your commitment to delivering accurate diagnostic test results to healthcare providers, a responsibility critical to ensuring that patients receive the most effective and timely treatment possible. In your lab, this means instituting a strong quality control (QC) program aimed at ensuring test performance and preventing the release of inaccurate results. The second driving force is the responsibility you have towards to running an efficient, cost-effective clinical lab operation. This entails the responsibility to manage resources efficiently, to stay within allotted budget guidelines, and to avoid unnecessary spending. The obvious assumption is that higher quality translates into higher costs (and bigger budgets). Look closer, however, and you’ll discover that the two forces outlined above are not necessarily opposed. As you’ll see below, increasing accuracy and reliability in a clinical testing lab — through investment in additional QC measures, such as high-quality third-party controls — can actually translate into decreasing costs. Staying within budget and providing reliable results can go hand in hand.
0 Comments Click here to read/write comments
3 Tips for Reducing Test Repeats and Lab Downtime
Category: M&S
Posted by
Eric Morreale, PhD on Nov 12, 2019 12:00:00 AM
The world of a clinical testing lab is one of high pressure. The pressure comes from clinicians who want their results as quickly as possible. It comes from directors and managers who demand efficiency. And most of all, it comes from a constant stream of samples that never lets up. In this era of largely automated instrumentation (operating almost always at full capacity), few things are more frustrating to lab personnel than errors that result in downtime, troubleshooting, and the need to rerun tests. Reportable results is the most important metric in a clinical lab. Any result that is not reportable translates into higher costs, more downtime, and a growing backlog. What can you do to reduce momentum-killing test failures and repeats while increasing your reportable result rates?
0 Comments Click here to read/write comments
For Clinical Labs: How to Ensure a Consistent Supply of High Quality Reference Materials
Category: M&S
Posted by
Eric Morreale, PhD on Oct 29, 2019 12:00:00 AM
Healthcare is — to put it mildly — unpredictable. You never know when testing demands will change due to a disease outbreak, or a shift in medical consensus. Thus, clinical labs operate with a basic mindset: be prepared.
0 Comments Click here to read/write comments
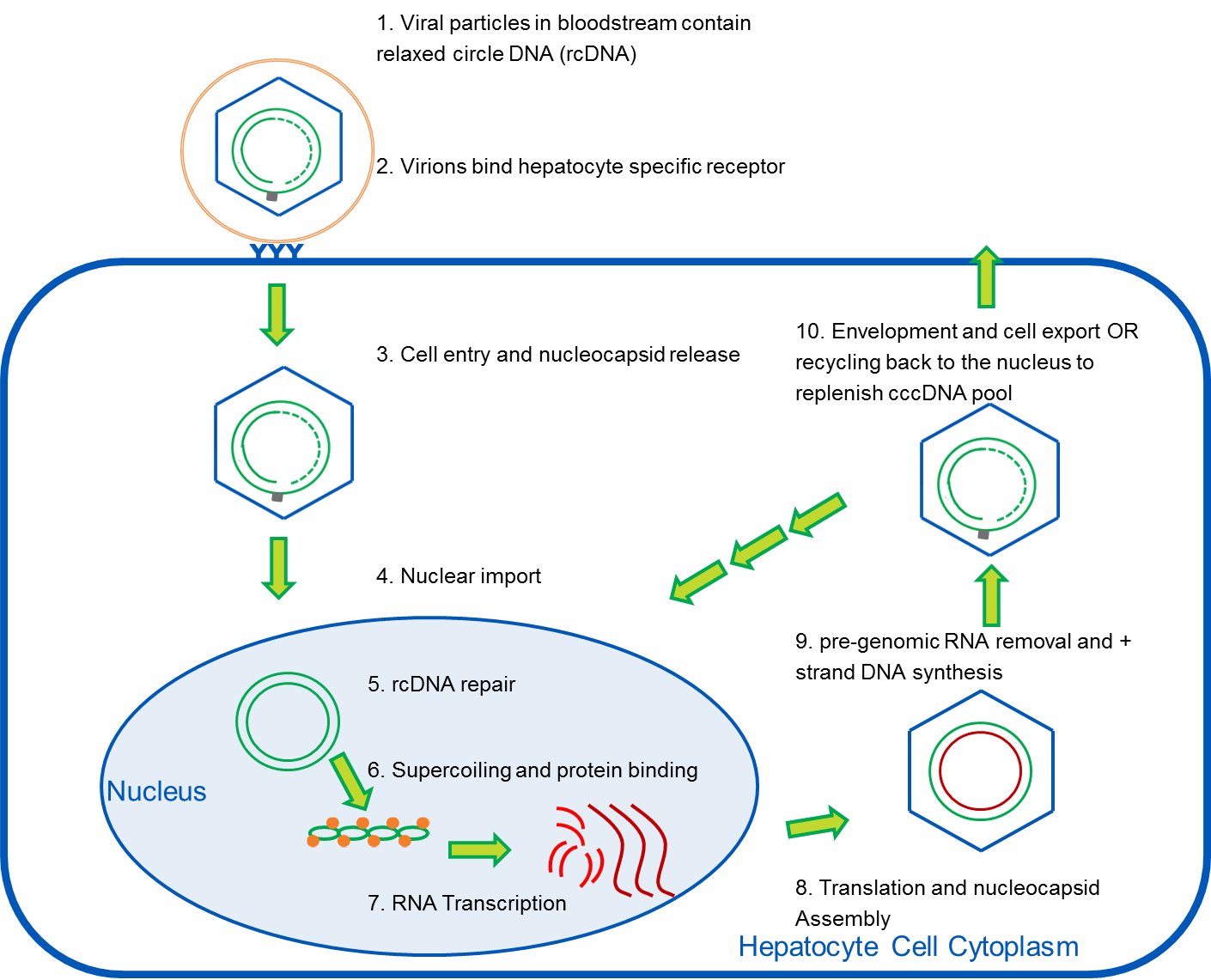
cccDNA and HBV: New Testing Methods May Allow for Earlier, Non-Invasive Detection of Hepatocellular Carcinoma
Category: Accuplex, Molecular & Serology
Posted by
Catherine Huang, PhD on Aug 23, 2018 12:00:00 AM
A recent study, published in the Journal of Molecular Diagnostics, describes a new, more sensitive Hepatitis B Virus (HBV) assay1. This study, led by Song-Mei Liu, MD, PhD, of the Center for Gene Diagnosis, Zhongnan Hospital of Wuhan University in China, is particularly exciting because the new assay can be used to diagnose hepatocellular carcinoma (HCC) at an earlier stage and to manage antiviral HBV treatments more effectively. It also highlights the way innovative molecular diagnostics can play a synergistic role with the development of new pharmaceutical therapeutics.
0 Comments Click here to read/write comments
Ebola Outbreak 2018: Diagnostics Again are Essential to Minimize Spread and to Control Disease
Category: Accuplex, reference materials, ebola, Molecular & Serology
Posted by
Catherine Huang, PhD on Jun 25, 2018 12:00:00 AM
On April 4th, 2018, a new outbreak of Ebola Virus Disease (EVD) occurred in Equateur Province in the Democratic Republic of the Congo. As of June 10th, there have been a total of 55 EVD cases and 28 deaths with a case fatality rate of 50.9%. Although the outbreak remains active, public health authorities have expressed cautious optimism because there have been no new cases in two of the three affected areas (Bikoro and Wangata zones) since May 17th, 2018 and the rate of new cases in the third affected zone (Iboko) has slowed.1
0 Comments Click here to read/write comments
Rapid and Early detection of Listeria to prevent food poisoning
Category: Accuplex, listeria, Molecular & Serology
Posted by
Vidya Murthy on Sep 12, 2017 12:00:00 AM
September is National Food Safety Education Month. This is also a time to return to school and learning, so it is a good time to learn about food safety in ready-to-eat and perishable foods often packed in school lunches. Listeria is one of the most common foodborne bacteria and is found in a wide range of foods; from ready-to-eat to produce and meat. The latest draft guidance released by the U.S. Food and Drug Administration, “Control of Listeria monocytogenes in Ready-To-Eat Foods,” supports ongoing efforts by industry and government agencies to reduce the risk of Listeria monocytogenes in ready-to-eat foods (1). The guidance includes recommendations for controls involving personnel, cleaning and maintenance of equipment, and sanitation, as well as for treatments that kill Listeria and prevent it from growing during storage of food between production and consumption. The guidance emphasizes the importance of routine testing for Listeria. Therefore, it is important to learn about the methods and reagents that can be used to detect Listeria to prevent foodborne outbreaks.
0 Comments Click here to read/write comments
Improve the Reliability of Your Zika Diagnostic Assays
Category: zika virus, Accuplex
Posted by
Vidya Murthy on May 9, 2017 12:00:00 AM
What is Zika? Zika virus (ZIKV) is an arbovirus that was first isolated in 1947 in a rhesus monkey from the Zika forest of Uganda. Until 2007, only 14 human cases were reported. The first large human outbreak occurred in 2007 (Yap Island, Micronesia, Pacific) followed by French Polynesia in 2013 that resulted in more than 30,000 cases. In early 2015, the virus came to international attention due to the outbreak in Brazil. Since then the epidemic has spread to more than 50 countries in the Caribbean, Central, and South America, causing tens of thousands of infections. ZIKV is transmitted to people primarily through the bite of an infected Aedes species mosquito (Ae. aegypti and Ae. albopictus). These are the same mosquitoes that spread dengue and chikungunya. ZIKV can also be transmitted sexually and during pregnancy from the infected mother to the fetus.
0 Comments Click here to read/write comments