Choose your Article Focus | NGS | Molecular & Serology
Molecular Diagnostics - Part 5: Analytical Sensitivity and Analytical Specificity Best Practices
Category: Molecular & Serology, Blog
Posted by
SeraCare Team on Feb 6, 2023 11:30:00 AM
The final article in our series about molecular diagnostics looks at best practices for analytical sensitivity and analytical specificity. Both are additional characteristics for laboratory-developed tests and modified FDA-cleared or approved tests.
0 Comments Click here to read/write comments
Molecular Diagnostics - Part 4: Reportable Range and Reference Interval Best Practices
Category: Molecular & Serology, Blog
Posted by
SeraCare Team on Dec 21, 2022 11:30:00 AM
Our series on molecular diagnostics continues with a look at best practices for two performance characteristics – reportable range and reference interval. These two characteristics need to be verified and documented for FDA-cleared or FDA-approved tests, laboratory-developed tests, and modified FDA-cleared or approved tests.
0 Comments Click here to read/write comments
Molecular Diagnostics - Part 3: Accuracy and Precision
Category: Molecular & Serology, Blog
Posted by
SeraCare Team on Oct 25, 2022 9:45:00 AM
We continue our series on molecular diagnostics. Previous articles covered: what you need to verify your molecular assay and the three major components of the verification process. FDA-cleared or FDA-approved tests have four performance characteristics that must be verified and documented: accuracy, precision, reportable range, and reference interval. Laboratory-developed tests and modified FDA-cleared or approved tests have two additional characteristics for verification (analytical sensitivity and analytical specificity). Now, it’s time to turn our attention to best practices for each of the performance characteristics. First up – accuracy and precision.
0 Comments Click here to read/write comments
Molecular Diagnostics - Part 2: Tackling the How
Category: Molecular & Serology, Blog
Posted by
SeraCare Team on Aug 30, 2022 11:48:55 AM
3 Important Stages of Assay Verification Molecular diagnostic tests are widely available in clinical microbiology laboratories now. It’s important to a lab’s success to understand the requirements before adding a new test. Protocols aren’t specified by regulators, so they vary by lab. The assay influences the controls and parameters, but the type of verification and its complexity are determined by lab directors. In a recent article, we took a look at what to verify. This is the first of several articles that will cover how to confirm a molecular assay performs as expected.
0 Comments Click here to read/write comments
How Low Can You Go? Performance at Lower Levels is Critical
Category: Molecular & Serology, Blog
Posted by
SeraCare Team on Jun 30, 2022 10:44:00 AM
Qualitative tests produce binary results, usually positive or negative. This presents a challenge for serological testing, since most of it is qualitative. Positive or negative results are determined in relation to a threshold value or cutoff. This cutoff, the line between positive and negative, is the medical decision point. It’s critical that this point is consistently acc urate at the lower limits of detection, where positive becomes negative. Without precision at the lower levels of positive results, laboratories may not feel confident in the results they present. Do they have a higher number of false positives or false negatives than they should?
0 Comments Click here to read/write comments
Molecular Diagnostics - Part 1: Know What to Verify
Category: Molecular & Serology, Blog
Posted by
SeraCare Team on Jun 28, 2022 2:52:33 PM
Adding a new molecular test to your lab? Make sure you understand the regulations that apply and the required performance characteristics. The following is the first in a series of articles on this topic that will include best practices. Before we dive into the how of verification for molecular diagnostic assays, let’s take a look at the what. Clinical laboratories performing an FDA-cleared or FDA-approved test must verify that the manufacturer’s performance characteristics can be met or exceeded Laboratories performing laboratory-developed tests (LDTs) and modified FDA-cleared or approved tests, must verify the same characteristics, plus determine analytical sensitivity and analytical specificity
0 Comments Click here to read/write comments
Key Strategies for Reducing Turnaround Time in Your Clinical Lab
Category: Molecular & Serology
Posted by
Eric Morreale, PhD on Mar 3, 2020 12:00:00 AM
They say good things come to those who wait, but when it comes to laboratory testing, faster is almost always better (assuming, of course, that accuracy is never compromised). The more rapidly that reportable results are generated, the quicker clinicians and patients can make decisions and embark on an effective treatment program. Furthermore, the more efficiently labs can run tests and generate results, the more they can accomplish. Faster turnaround times (TAT) can free up staff and resources for other activities, like growing the overall test menu. And let’s not forget the reputation factor. Labs want to be the reliable go-to for the clinicians they serve. They want to be trusted for their accuracy, professionalism, and speed.
0 Comments Click here to read/write comments
4 Best Practices for QC in a Clinical Testing Lab to Ensure Accurate Results
Category: Molecular & Serology
Posted by
Eric Morreale, PhD on Feb 18, 2020 12:00:00 AM
What is a clinical laboratory director’s worst nightmare? That’s an easy one. The thought that, somehow, an inaccurate testing result has escaped their lab and convinced an unsuspecting clinician to make a poor patient care decision. So much of modern medical care is grounded in treatment decisions based on diagnostic testing results. According to one review of the data, 98 percent of in-patient populations get lab tests. False negatives can delay life-saving and life-improving patient care. False positives can cause patients to undergo unnecessary treatment, which can result in needless psychological, physical, and financial distress. No wonder lab directors are troubled by the possibility, no matter how small, of reporting inaccurate results - critical patient care decisions hang in the balance. (Not to mention the professional reputations of lab directors, the reputations of their labs and hospitals, and the trust of patients and clinicians.) To prevent errors in testing and reporting, quality control is a must. You need to be confident every time you report a result that your assays are working the way they are intended. Here are four tips for optimizing quality control to ensure accurate results in your clinical lab:
0 Comments Click here to read/write comments
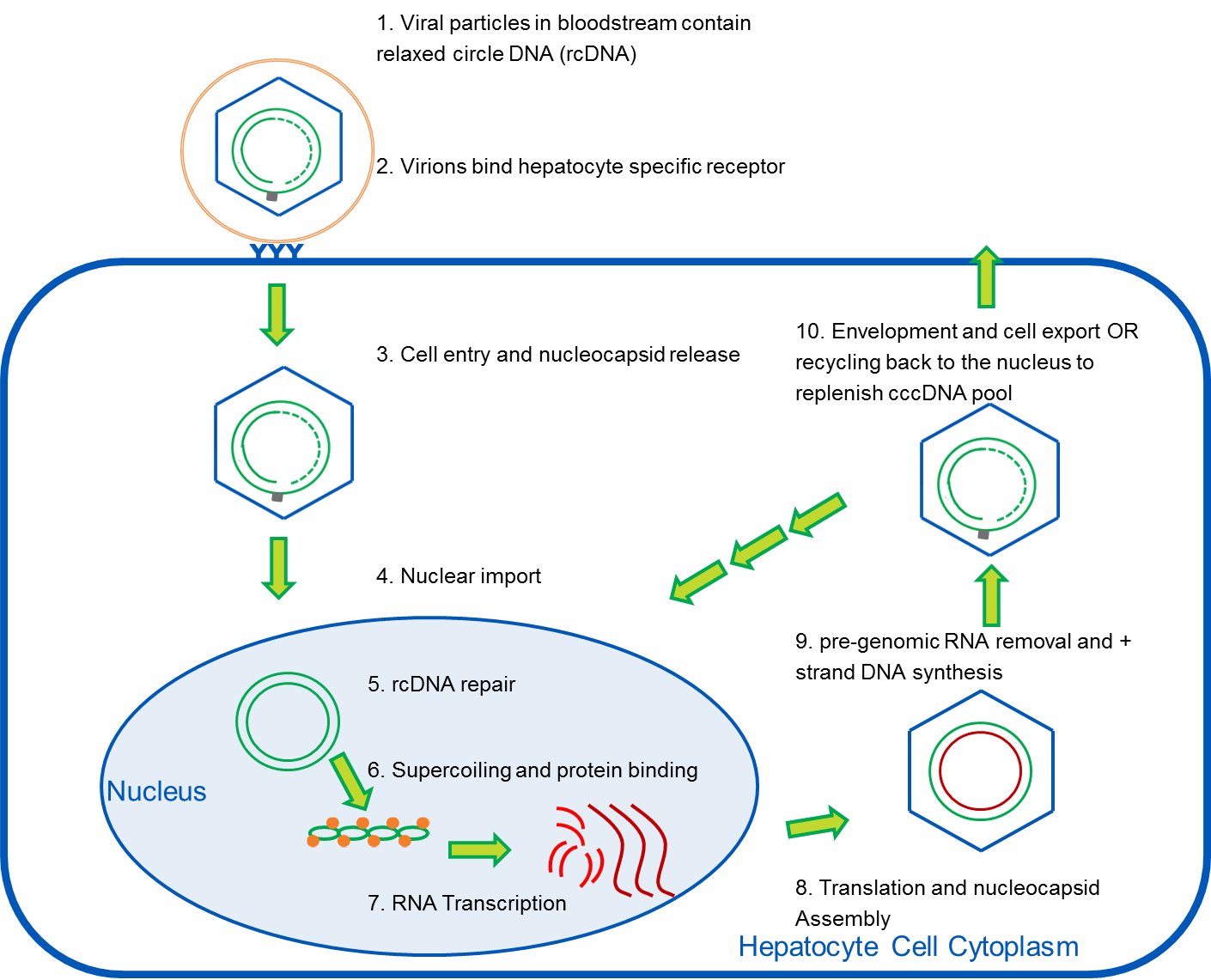
cccDNA and HBV: New Testing Methods May Allow for Earlier, Non-Invasive Detection of Hepatocellular Carcinoma
Category: Accuplex, Molecular & Serology
Posted by
Catherine Huang, PhD on Aug 23, 2018 12:00:00 AM
A recent study, published in the Journal of Molecular Diagnostics, describes a new, more sensitive Hepatitis B Virus (HBV) assay1. This study, led by Song-Mei Liu, MD, PhD, of the Center for Gene Diagnosis, Zhongnan Hospital of Wuhan University in China, is particularly exciting because the new assay can be used to diagnose hepatocellular carcinoma (HCC) at an earlier stage and to manage antiviral HBV treatments more effectively. It also highlights the way innovative molecular diagnostics can play a synergistic role with the development of new pharmaceutical therapeutics.
0 Comments Click here to read/write comments
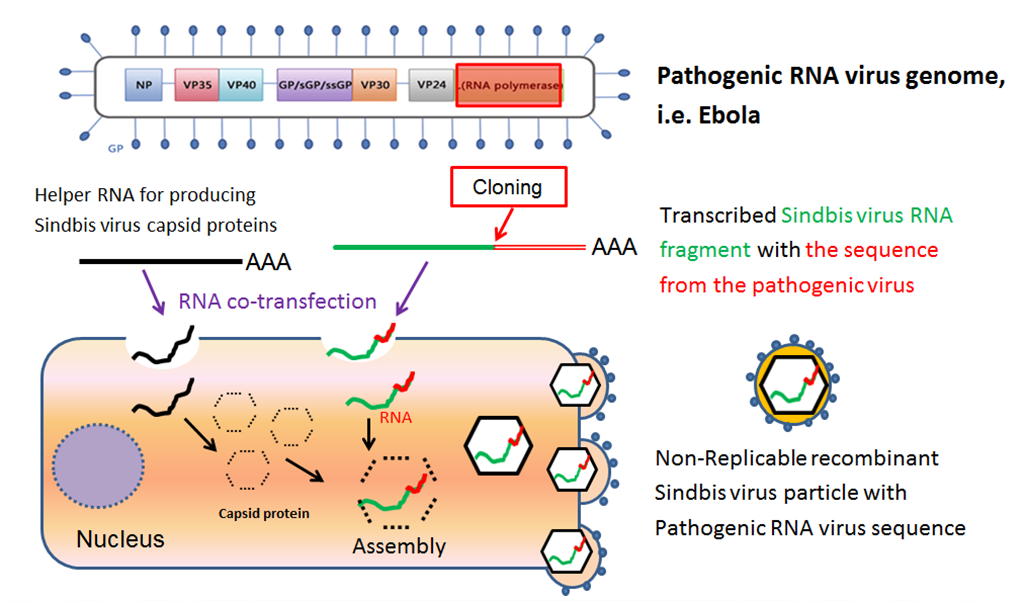
Ebola Outbreak 2018: Diagnostics Again are Essential to Minimize Spread and to Control Disease
Category: Accuplex, reference materials, ebola, Molecular & Serology
Posted by
Catherine Huang, PhD on Jun 25, 2018 12:00:00 AM
On April 4th, 2018, a new outbreak of Ebola Virus Disease (EVD) occurred in Equateur Province in the Democratic Republic of the Congo. As of June 10th, there have been a total of 55 EVD cases and 28 deaths with a case fatality rate of 50.9%. Although the outbreak remains active, public health authorities have expressed cautious optimism because there have been no new cases in two of the three affected areas (Bikoro and Wangata zones) since May 17th, 2018 and the rate of new cases in the third affected zone (Iboko) has slowed.1
0 Comments Click here to read/write comments





.png)