This is a third blog in a series on RNA fusions, this time focusing on how the FFPE Fusion RNA materials are used as RNA extraction controls.
Gene Fusion, an Emerging Biomarker
Gene fusions as a biomarker began with the discovery of the Philadelphia Chromosome (BCR-ABL fusion gene) for monitoring chronic myelogenous leukemia (CML) patients, understanding of the underlying pathology, and the development of clinically successful Tyrosine Kinase Inhibitors (TKI) such as Imatinib (Gleevec). New targeted therapies have since emerged based on fusion genes (ALK, RET, NTRK) making the analysis of gene fusions an important part of clinical management of hematologic and solid cancers. There are over 40 recruiting trials in the Clinical Trials database involving gene fusion biomarkers1.
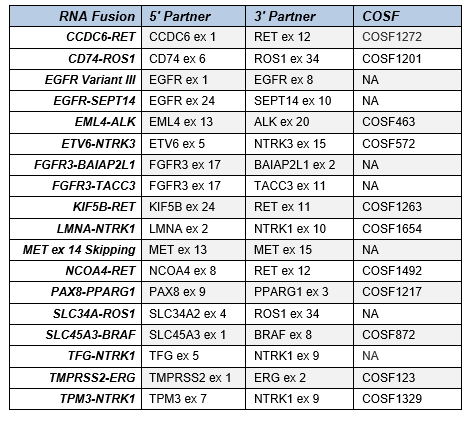
The increase of actionable gene fusions for solid tumors has prompted a need for using multiplexed and higher throughput diagnostic tools. However, genomic breakpoints are intrinsically challenging because these are detectable only when present and their prevalence is often of low abundance. Therefore, sourcing samples as positive run control or full process sample control in targeted NGS RNA fusion assays is difficult. Yet, such samples are important in clinical patient testing. SeraCare has developed the Seraseq® FFPE Fusion RNA reference materials (v4) with 18 fusion targets (Table 1) covering a broad spectrum of solid tumors, making them ideal to support assay validation, monitoring longitudinal assay performance, and routine clinical tests2.
Table 1. List of 18 clinically-actionable fusion targets in the Seraseq® FFPE Fusion RNA Reference Material (v4).
We report here a multi-laboratory evaluation (Site A, B, C & D) of the latest release of the Seraseq FFPE Fusion RNA Reference Material (v4), that describes RNA extraction efficiency and quantitative detection of all 18 fusion targets using different NGS chemistries and platforms. The results support the utility of the pan-cancer Seraseq FFPE Fusion RNA Reference Material to support end-to-end targeted NGS assay development, validation and guiding cancer patient management.
Multiplexed and Quantitated FFPE Rreference Material
The Seraseq FFPE Fusion RNA Reference Material (v4) is a contrived reference standard consisting of engineered cells that have been formalin-fixed and paraffin-embedded (FFPE) to create an FFPE block, which is then sectioned into 10 µm curls. The reference standard is constructed from a pool of in vitro transcribed fusion RNA constructs and the RNA pool is transfected into background GM24385 human cell line WT. Every fusion RNA transcript contains approximately 400 bp of each fusion partner and ~37 bp poly-A tail. The number of fusion RNA transcripts in the final material is quantitated using ddPCR targeting each transcribed transcript. The translocation events are not present at the DNA level.
FFPE Extraction Results
The molecular diagnostic NGS workflow is complex, therefore having access to multiplexed and well-characterized reference samples is an important supplement to patient specimens. High-quality reference material enables comprehensive assay performance evaluation across several clinically-actionable variants at well-defined expression levels or allelic frequencies. Tissue specimens for solid tumor analysis are commonly preserved in FFPE blocks which readily allow histopathological scoring; however, this preservation adds layers of complexity to the molecular diagnostic NGS workflow3. One such layer is the initial RNA extraction from the FFPE material. Reference material of known character can help harmonize the pre-analytic variable of extraction methodology. Table 2 below highlights the results of RNA extractions by four independent laboratories (Site A, B, C & D) as well as internal development data from various extraction and quantification methodologies.
Table 2: Yield per 10 µm section of the Seraseq® FFPE Fusion RNA Reference Material (v4) performed at different laboratories (Site A, B, C & D) using various FFPE extraction and RNA quantification methods.
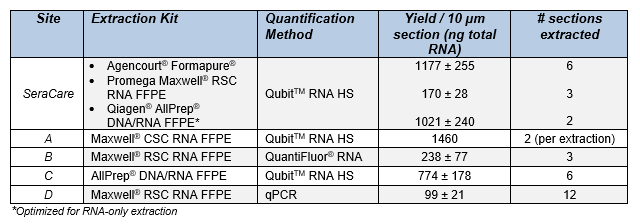
The results show consistency in yield per extraction kit, hence this reference material is highly suitable for longitudinal monitoring of patient sample pre-analytics in a molecular diagnostic workflow for solid tumors.
Performance on Amplicon- and Hybrid Capture-based Targeted NGS Panels
Another layer of complexity introduced by FFPE specimen preservation is sample degradation, which together with cellular heterogeneity, convolute determination of assay performance characteristics.
We have previously reported a multi-laboratory study of purified RNA reference standards using amplicon- and hybrid capture-based targeted NGS panels with the Seraseq Fusion RNA Mix (v4)4 which shows a consistent performance by all NGS platforms in detecting all 18 fusion targets in the reference sample. A similar multi-laboratory evaluation of the FFPE-based reference standard was performed with three prepared FFPE blocks of the Seraseq FFPE Fusion RNA Reference Material (v4). The results show consistent RNA fusion detection across the different FFPE blocks (Figure 1).
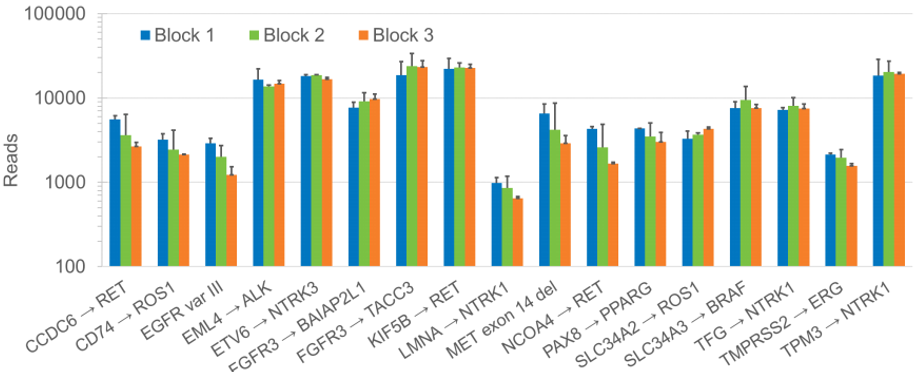
Figure 1: Plot showing the number of reads supporting RNA fusion targets analyzed by the Oncomine™ Focus Assay (average and 1 SD of two runs using 50 ng input of RNA extracted from three different FFPE blocks). Note: EGFR-SEPT14 detection isn’t included in assay design.
Conclusion
Translocations are an increasingly important biomarker for solid tumor disease profiling and diagnosis. However, these are intrinsically challenging to detect since these are often rare events and can pose technical challenges for the wet and dry lab elements of the analysis. The Seraseq FFPE Fusion RNA Reference Material (v4) has been shown to provide consistent assessment and longitudinal monitoring of the complete molecular diagnostic NGS workflow for the detection of 18 clinically actionable RNA fusions common in many metastatic solid tumors.
References:
- Recruiting Studies - gene fusion. ClinicalTrials.gov Available at: https://clinicaltrials.gov/ (Accessed: 6th March 2020)
- Ruminski-Lowe, D. et al. Consistent performance of highly multiplexed RNA fusion reference materials across different NGS assays in a multi-lab study. in Cancer Research 1709 (2019). doi:DOI:10.1158/1538-7445.AM2019-1709
- Bonnet, E. et al. Performance comparison of three DNA extraction kits on human whole-exome data from formalin-fixed paraffin-embedded normal and tumor samples. PLoS One 13, e0195471 (2018). PMID: 29621323
- Anfora, A. Multi-Lab Study of Fusion RNA Reference Standards for Targeted NGS. Available at: https://blog.seracare.com/. (Accessed: 6th March 2020)




