Choose your Article Focus | NGS | Molecular & Serology
Diagnostic Testing Schemes for NTRK Cancers: All Roads Lead to NGS
Category: SeraSeq, NTRK, NGS, RNA fusion
Posted by
Russell Garlick, PhD on Feb 12, 2020 12:00:00 AM
New treatment options for cancer patients with neurotrophic tyrosine kinase (NTRK) rearrangements are a tremendous success, demonstrating first-hand the importance of precision diagnostics. The FDA granted accelerated approval of Bayer's VITRAKVI (larotrectinib) for adult and pediatric solid tumors containing NTRK fusions1. The approval was one of the first tissue agnostic approvals based on genotyping results, and included patients with unresectable or metastatic cancer. For 12 cancer types, the overall response rate was 75% with 22% complete response and 53% partial response. In addition to VITRAKVI, Genentech's entrectinib has a "breakthrough" status and is being evaluated for the potential treatment of advanced or metastatic tumors that harbor NTRK or ROS1 gene rearrangements.
0 Comments Click here to read/write comments
Multi-Lab Study of Fusion RNA Reference Standards for Targeted NGS
Category: NTRK, NGS, RNA fusion, reference materials, AACR
Posted by
Andrew Anfora, PhD on Jan 28, 2020 12:00:00 AM
Sourcing assay validation samples as positive run controls or workflow controls in targeted NGS RNA fusion assays remains a challenge today. This is further exacerbated with clinical labs looking to provide validated NGS assays for patient stratification in a host of new drugs in clinical trials or newly approved targeting fusion genes, such as NTRK genes (Larotrectinib, Loxo/Bayer) and Entrectinib (Genentech/Roche) for rare cancers in adult and pediatric patients, and RET (Loxo/Lilly) for lung cancer. SeraCare produces several RNA fusion reference materials. This article describes the development and multi-laboratory evaluation of a pan-cancer multiplexed Fusion RNA reference standard for analysis of clinically relevant fusion genes in solid tumors. The evaluation was conducted at 5 different laboratories on different NGS platforms (amplicon- and hybridization capture-based) as well as at different RNA inputs within a platform. Results highlight the utility of this Fusion RNA reference material to support clinical NGS assays as positive controls in solid tumor cancer patient stratification for many of these fusion-based targeted therapies.
0 Comments Click here to read/write comments
Presenting NTRK Reference Materials for Global Assay Standardization at AACR 2019
Category: SeraSeq, NTRK, RNA fusion, AACR
Posted by
Catherine Huang, PhD on Apr 8, 2019 12:00:00 AM
On the last morning of AACR 2019, I had the privilege of presenting a poster together with my colleague, Sebastian Bender from Bayer AG, in Berlin. Because of this, I didn’t have a chance to attend any talks, but I still wanted to finish out my blog series with highlights from each day of the conference.
0 Comments Click here to read/write comments
NTRK Fusion Testing for New Therapies
Category: NTRK, cancer, RNA fusion
Posted by
Omo Clement, PhD on Feb 6, 2019 12:00:00 AM
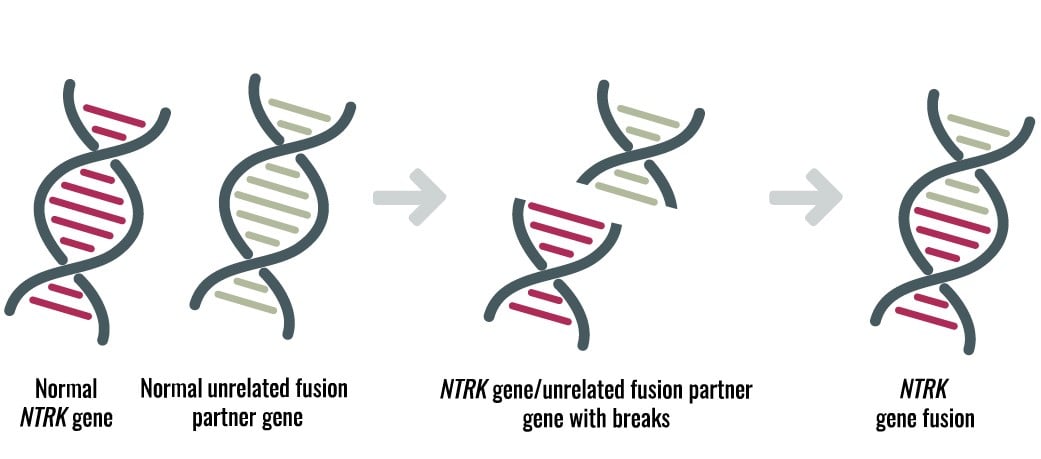
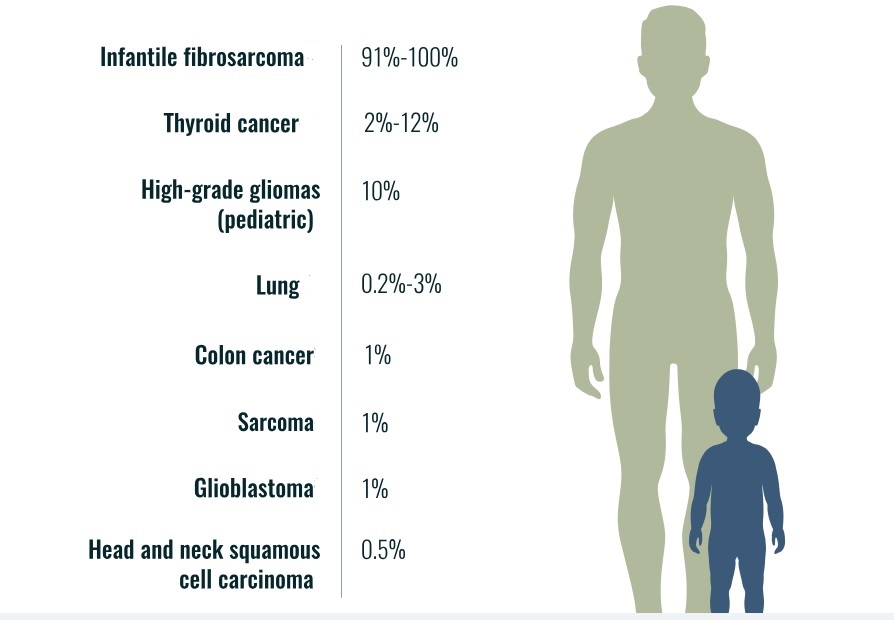
Neurotrophic tyrosine receptor kinases (NTRK) can become abnormally fused to other genes resulting in growth signals that can lead to cancer in many organs of the human body. TRK gene fusion-based cancers are rare but present in pediatric and adult cancers such as lung, thyroid, colon, etc. (see, e.g., Figure 1).
0 Comments Click here to read/write comments