Choose your Article Focus | NGS | Molecular & Serology
Keys to Better Liquid Biopsy Assay Sensitivity – AMP Corporate Workshop Video
Category: AMP, liquid biopsy, NGS, ctDNA
Posted by
Sam Blier on Jan 24, 2019 12:00:00 AM
“So as everyone here is aware, I’m sure, detection of circulating tumor DNA is challenging. There’s very little of it, to start with.” Hardly a revolutionary statement by Tony Godfrey, PhD, (Associate Chair, Surgical Research and Associate Professor of Surgery, Boston University School of Medicine) but an important acknowledgement from a leading expert of the difficulty faced by laboratorians
0 Comments Click here to read/write comments
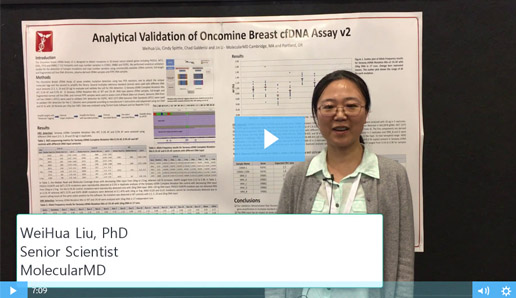
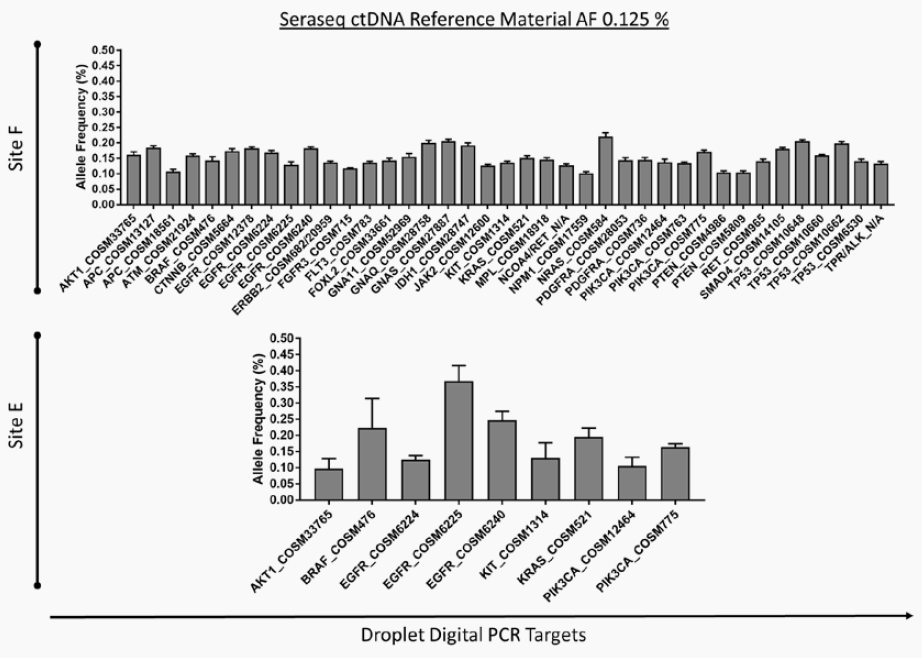
Two Must-See Liquid Biopsy Poster Videos From AMP 2018
Category: AMP, SeraSeq, liquid biopsy, cfDNA, ctDNA
Posted by
Sam Blier on Dec 7, 2018 12:00:00 AM
Of the many fantastic posters presented at AMP’s Annual Meeting in San Antonio, two concerning NGS-based liquid biopsy assays stood out. Both presenters described how their organizations are working to reliably detect pathogenic variants at extremely low allele frequencies – efforts critical to the clinical adoption of NGS-based liquid biopsy assays.
0 Comments Click here to read/write comments
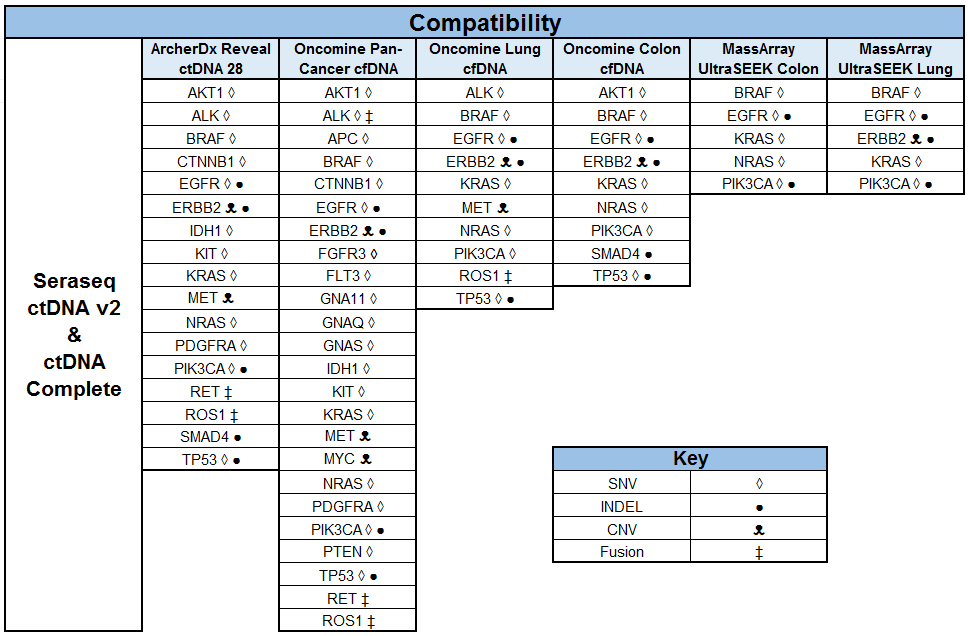
Building and Implementing Liquid Biopsy Assays with the Industry’s Most Patient-Like Reference Materials
Category: SeraSeq, liquid biopsy, NGS, reference materials
Posted by
Omo Clement, PhD on Oct 24, 2018 12:00:00 AM
SeraCare’s clinical genomics technologies are developed to address challenges faced across the spectrum of NGS assays. From early development of assays – either IVD assay manufacturers or clinical labs building their own LDTs - there is a scarcity of characterized, complex, difficult variants to ensure the assay can robustly detect all the critical genomic variants in a patient sample. Using our highly characterized, reproducible, and GMP-grade NGS standards, laboratories have a wide range of analytical and clinical validation tools to deeply characterize assay performance such as LOD, linearity, specificity, sensitivity, and reproducibility.
0 Comments Click here to read/write comments
To Realize the Potential of Liquid Biopsies, Focus on Higher Quality Research and Clinical Data Sets
Category: SeraSeq, liquid biopsy
Posted by
Dan Brudzewsky on Oct 15, 2018 12:00:00 AM
Liquid biopsy requires better standardization to realize all the new possibilities for studying metastasis, heterogenicity, treatment efficacy, and disease recurrence. Furthermore, it is critical for clinicians to have confidence in liquid biopsy data to diagnose and treat patients. This is only achievable when consistent and high-quality data is generated at research and all clinical centers. The Liquid Biopsies course at EMBL Advanced Training Centre provides a unique practical training in best practices and pitfalls on the complete liquid biopsy workflow, from sample preparation to data analysis. The course is targeted for clinical laboratory and research scientists interested in learning all aspects of liquid biopsy testing.
0 Comments Click here to read/write comments
How A New Generation of ctDNA Reference Standards Are Enabling the Promise of Precision Medicine
Category: SeraSeq, liquid biopsy, NGS, cancer, ctDNA
Posted by
Omo Clement, PhD on Jun 14, 2018 12:00:00 AM
An important goal in cancer disease management is early detection. When detected early, disease progression can be significantly mitigated with a plethora of options (targeted therapy, chemotherapy, surgery, etc.) available to medical practitioners, to afford progression free survival and a higher quality of life. A great promise of liquid biopsies is the possibility of early detection of cancer long before clear evidence of lesions and tumor growth observable by imaging or other techniques.1 As proxy for solid tissue biopsies, plasma-based liquid biopsy application is rapidly gaining traction in cancer disease diagnosis, progression, monitoring, and in predicting resistance to treatment options.2
0 Comments Click here to read/write comments
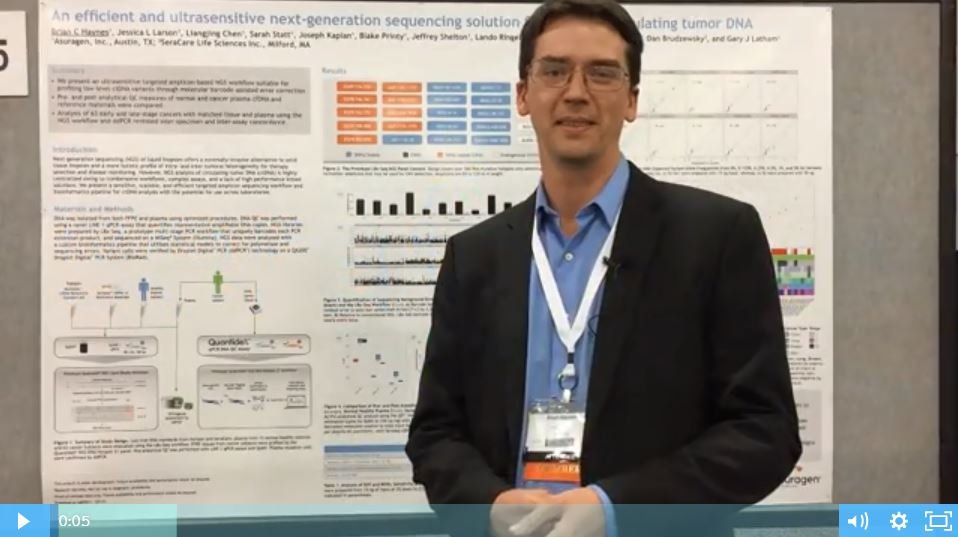
An Efficient and Ultrasensitive NGS Solution for Profiling ctDNA [Poster Talk Video]
Category: liquid biopsy, NGS, ctDNA
Posted by
Trevor Brown on Mar 5, 2018 12:00:00 AM
SeraCare Customer Poster Talk Video with Data Presented by Asuragen Next-generation sequencing (NGS) of liquid biopsies offers a minimally invasive alternative to solid tissue biopsies and a more holistic profile of intra- and inter-tumoral heterogeneity for therapy selection and disease monitoring. Watch the video and download this free poster to learn:
0 Comments Click here to read/write comments
An Efficient and Ultrasensitive NGS Solution for Profiling ctDNA [Poster Talk Video]
Category: liquid biopsy, NGS, ctDNA
Posted by
Trevor Brown on Feb 28, 2018 12:00:00 AM
SeraCare Customer Poster Talk Video with Data Presented by Asuragen Next-generation sequencing (NGS) of liquid biopsies offers a minimally invasive alternative to solid tissue biopsies and a more holistic profile of intra- and inter-tumoral heterogeneity for therapy selection and disease monitoring. Watch the video and download this free poster to learn: How reference materials that commute to the target sample type can help to optimize ctDNA profiling technology Why the Seraseq ctDNA v2 Reference Material most closely resembles native ctDNA in amplifiablity and molecular diversity How highly patient-like reference materials allowed confident quantification of trace levels of ctDNA
0 Comments Click here to read/write comments
Accelerating Liquid Biopsy Assay Development
Category: liquid biopsy, Assay Development, ctDNA, reference materials
Posted by
Sam Blier on Sep 8, 2017 12:00:00 AM
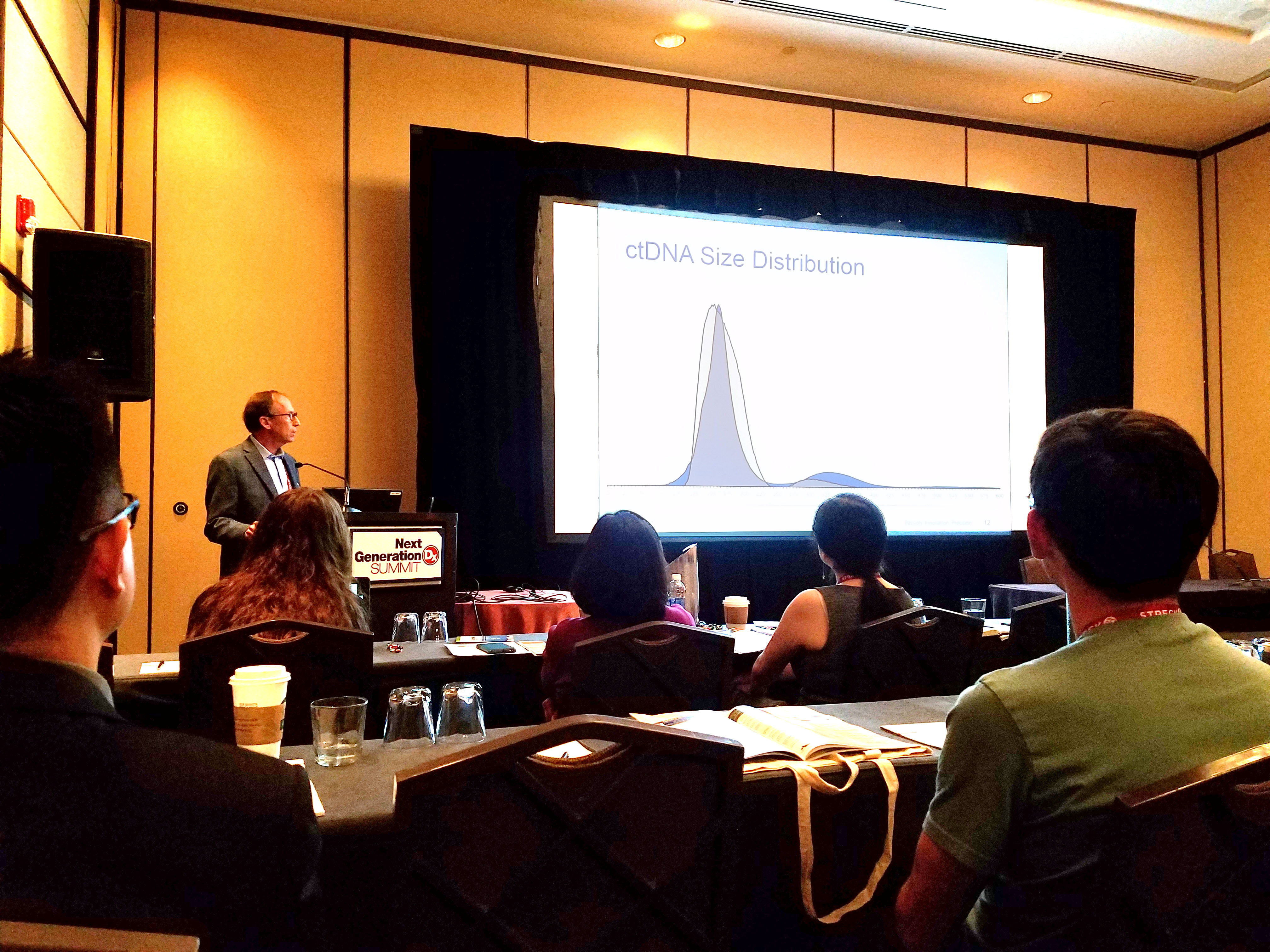
At the 2017 Next Generation Dx Summit in Washington, DC, our CSO, Russell Garlick, PhD, presented a workshop on accelerating liquid biopsy assay development. He has worked closely with a variety of groups in the liquid biopsy space that are developing and validating circulating tumor DNA (ctDNA) assays. He highlighted some common challenges facing the field, and explained how SeraCare has been using these collaborations to develop QC tools specifically for ensuring the robustness of these cutting-edge tests.
0 Comments Click here to read/write comments
How Many Target Copies Are Present in Your Plasma DNA Sample?
Category: liquid biopsy, cancer, Assay Development, ctDNA
Posted by
Dale Yuzuki on Jun 15, 2017 12:00:00 AM
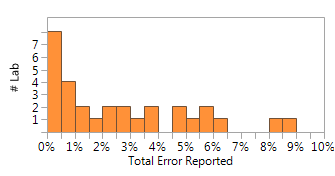
The field of circulating tumor DNA analysis (ctDNA, also sometimes called in a larger context “liquid biopsy”) holds great promise for monitoring response to cancer treatment, assisting therapeutic choice, monitoring recurrence, and for pre-cancer screening. As such there is a great amount of assay development and ongoing clinical trials; at ClinicalTrials.gov searching for the term "Circulating DNA" you can find over 180 open clinical trials for a wide range of tumor types and interventions.
0 Comments Click here to read/write comments
What lessons for liquid biopsy have been learned from fetal aneuploidy testing?
Category: liquid biopsy, NIPT, ctDNA
Posted by
Russell Garlick, PhD on Sep 30, 2016 12:00:00 AM
Non-invasive prenatal screening (NIPS) is currently offered in over 80 countries, covering over 80 million annual births, with an estimated volume of over one million screening tests performed annually. First offered in 2011, there has been rapid adoption of these genomic tests in the marketplace.
0 Comments Click here to read/write comments