Choose your Article Focus | NGS | Molecular & Serology
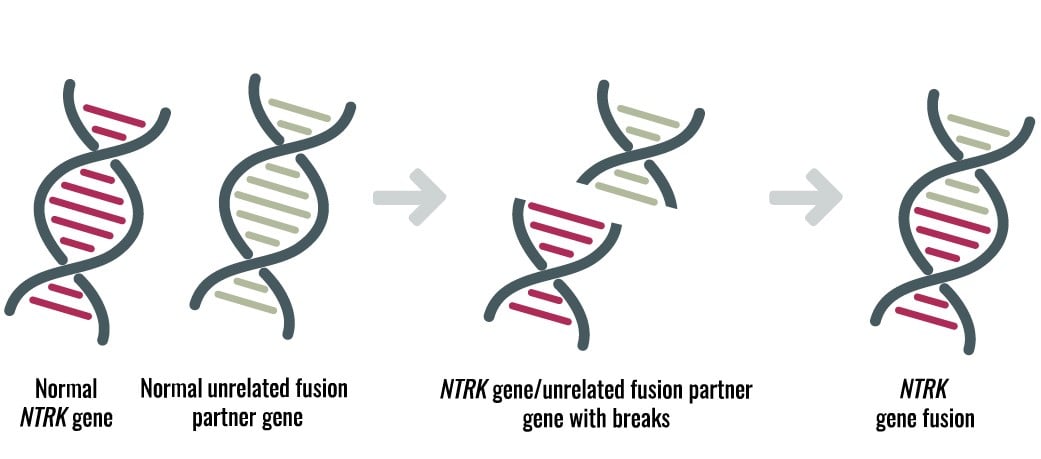
Diagnostic Testing Schemes for NTRK Cancers: All Roads Lead to NGS
Category: SeraSeq, NTRK, NGS, RNA fusion
Posted by
Russell Garlick, PhD on Feb 12, 2020 12:00:00 AM
New treatment options for cancer patients with neurotrophic tyrosine kinase (NTRK) rearrangements are a tremendous success, demonstrating first-hand the importance of precision diagnostics. The FDA granted accelerated approval of Bayer's VITRAKVI (larotrectinib) for adult and pediatric solid tumors containing NTRK fusions1. The approval was one of the first tissue agnostic approvals based on genotyping results, and included patients with unresectable or metastatic cancer. For 12 cancer types, the overall response rate was 75% with 22% complete response and 53% partial response. In addition to VITRAKVI, Genentech's entrectinib has a "breakthrough" status and is being evaluated for the potential treatment of advanced or metastatic tumors that harbor NTRK or ROS1 gene rearrangements.
0 Comments Click here to read/write comments
Presenting NTRK Reference Materials for Global Assay Standardization at AACR 2019
Category: SeraSeq, NTRK, RNA fusion, AACR
Posted by
Catherine Huang, PhD on Apr 8, 2019 12:00:00 AM
On the last morning of AACR 2019, I had the privilege of presenting a poster together with my colleague, Sebastian Bender from Bayer AG, in Berlin. Because of this, I didn’t have a chance to attend any talks, but I still wanted to finish out my blog series with highlights from each day of the conference.
0 Comments Click here to read/write comments
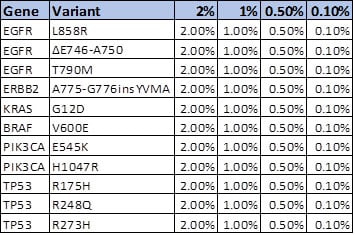
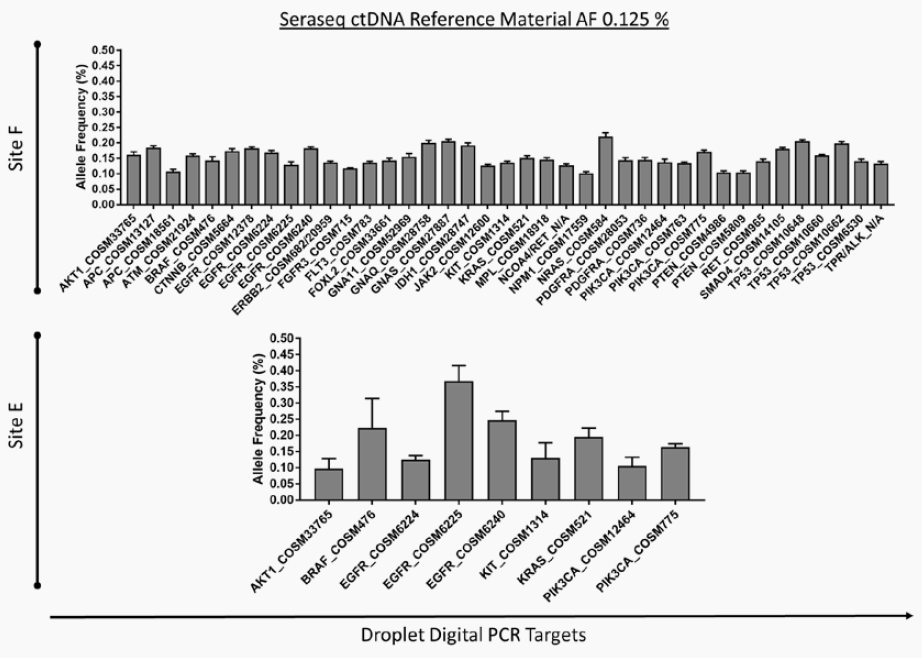
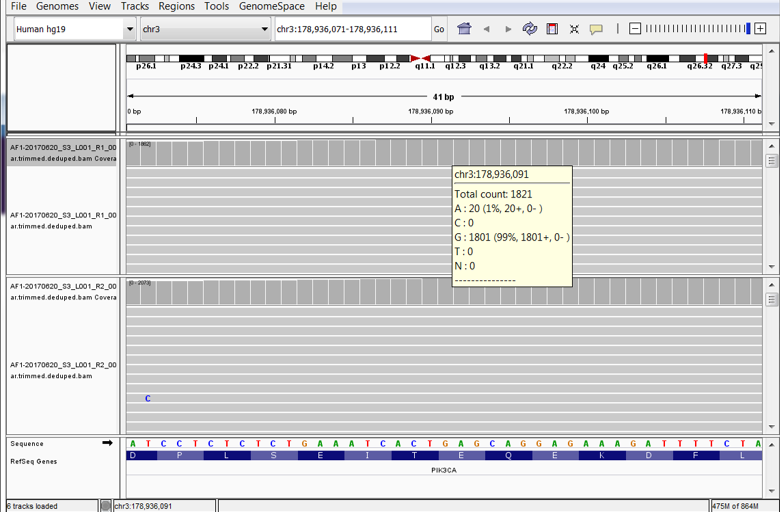
Customer Data: Use of Seraseq ctDNA Reference Samples to Validate Detection of Low Frequency Variants in a cfDNA-based NGS Lung Cancer Panel
Category: AMP, SeraSeq, cfDNA, Lung Cancer, ctDNA
Posted by
Omo Clement, PhD on Feb 14, 2019 12:00:00 AM
At the recently-concluded 2018 AMP Meeting, researchers at the New York Presbyterian Hospital (NYPH) and Weill Cornell Medical Center (WCMC) presented a poster1 on the validation of an Oncomine™ cell-free DNA Lung Assay using ctDNA NGS standards developed by SeraCare (Seraseq® ctDNA v2 Reference Materials),2
0 Comments Click here to read/write comments
Two Must-See Liquid Biopsy Poster Videos From AMP 2018
Category: AMP, SeraSeq, liquid biopsy, cfDNA, ctDNA
Posted by
Sam Blier on Dec 7, 2018 12:00:00 AM
Of the many fantastic posters presented at AMP’s Annual Meeting in San Antonio, two concerning NGS-based liquid biopsy assays stood out. Both presenters described how their organizations are working to reliably detect pathogenic variants at extremely low allele frequencies – efforts critical to the clinical adoption of NGS-based liquid biopsy assays.
0 Comments Click here to read/write comments
Building and Implementing Liquid Biopsy Assays with the Industry’s Most Patient-Like Reference Materials
Category: SeraSeq, liquid biopsy, NGS, reference materials
Posted by
Omo Clement, PhD on Oct 24, 2018 12:00:00 AM
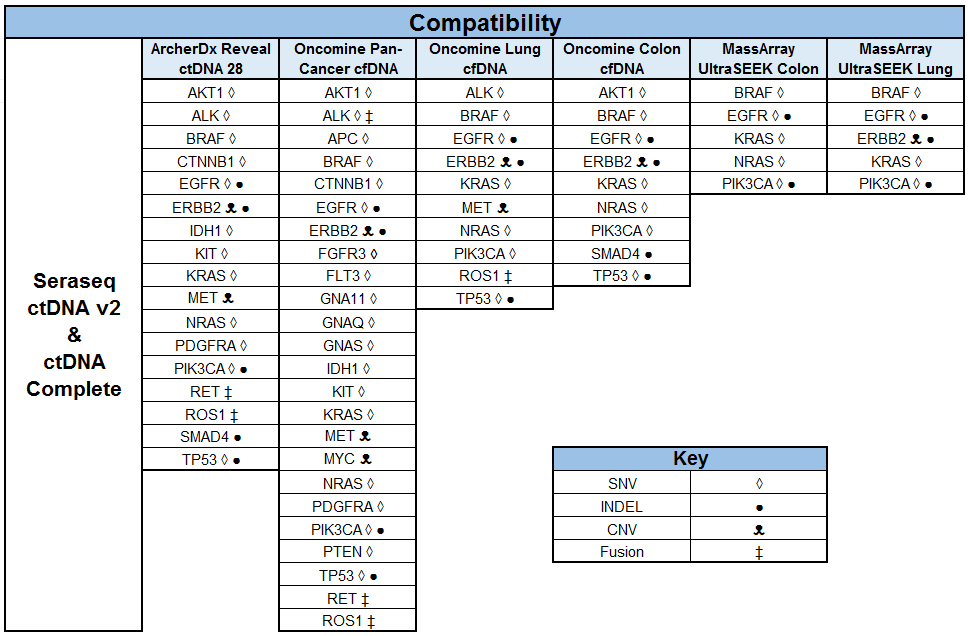
SeraCare’s clinical genomics technologies are developed to address challenges faced across the spectrum of NGS assays. From early development of assays – either IVD assay manufacturers or clinical labs building their own LDTs - there is a scarcity of characterized, complex, difficult variants to ensure the assay can robustly detect all the critical genomic variants in a patient sample. Using our highly characterized, reproducible, and GMP-grade NGS standards, laboratories have a wide range of analytical and clinical validation tools to deeply characterize assay performance such as LOD, linearity, specificity, sensitivity, and reproducibility.
0 Comments Click here to read/write comments
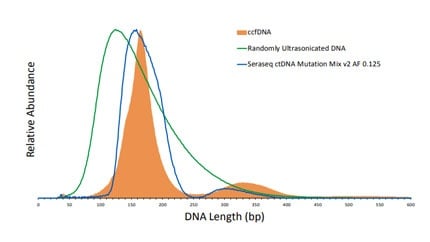
To Realize the Potential of Liquid Biopsies, Focus on Higher Quality Research and Clinical Data Sets
Category: SeraSeq, liquid biopsy
Posted by
Dan Brudzewsky on Oct 15, 2018 12:00:00 AM
Liquid biopsy requires better standardization to realize all the new possibilities for studying metastasis, heterogenicity, treatment efficacy, and disease recurrence. Furthermore, it is critical for clinicians to have confidence in liquid biopsy data to diagnose and treat patients. This is only achievable when consistent and high-quality data is generated at research and all clinical centers. The Liquid Biopsies course at EMBL Advanced Training Centre provides a unique practical training in best practices and pitfalls on the complete liquid biopsy workflow, from sample preparation to data analysis. The course is targeted for clinical laboratory and research scientists interested in learning all aspects of liquid biopsy testing.
0 Comments Click here to read/write comments
If I Call Out of Tune, Would Mutations Stand Up and Walk Out on Me?
Posted by
Matthew Butler on Aug 9, 2018 12:00:00 AM
One of several important steps in next-generation sequencing (NGS) is tuning the many options provided by mutation callers. Providing values for options configures the signal to noise ratio of the impending mutation calls. In theory, providing values that increase the stringency of mutation calls will reduce the number of false positive calls and thus enrich for true positives. In practice, increasing stringency can eliminate true positives.
0 Comments Click here to read/write comments
How A New Generation of ctDNA Reference Standards Are Enabling the Promise of Precision Medicine
Category: SeraSeq, liquid biopsy, NGS, cancer, ctDNA
Posted by
Omo Clement, PhD on Jun 14, 2018 12:00:00 AM
An important goal in cancer disease management is early detection. When detected early, disease progression can be significantly mitigated with a plethora of options (targeted therapy, chemotherapy, surgery, etc.) available to medical practitioners, to afford progression free survival and a higher quality of life. A great promise of liquid biopsies is the possibility of early detection of cancer long before clear evidence of lesions and tumor growth observable by imaging or other techniques.1 As proxy for solid tissue biopsies, plasma-based liquid biopsy application is rapidly gaining traction in cancer disease diagnosis, progression, monitoring, and in predicting resistance to treatment options.2
0 Comments Click here to read/write comments
FAQ: What to do when your NGS assay fails to detect a variant contained in Seraseq Reference Materials?
Posted by
Catherine Huang, PhD on Jun 11, 2018 12:00:00 AM
Highly multiplexed reference materials are particularly valuable when developing and optimizing new NGS assays because they allow you to evaluate the performance of your assay across a large number of variants including different variant types (SNVs, indels, homopolymeric variants, etc.) and contexts. However, it can be frustrating when a variant in the reference material is not detected, or not detected at the expected variant allele frequency. Troubleshooting such issues can give new insight into the performance of the assay. Here we share some stories from Seraseq™ users where the lack of detection of one or more variants at the expected levels helped them improve their assay or set more appropriate QC thresholds.
0 Comments Click here to read/write comments
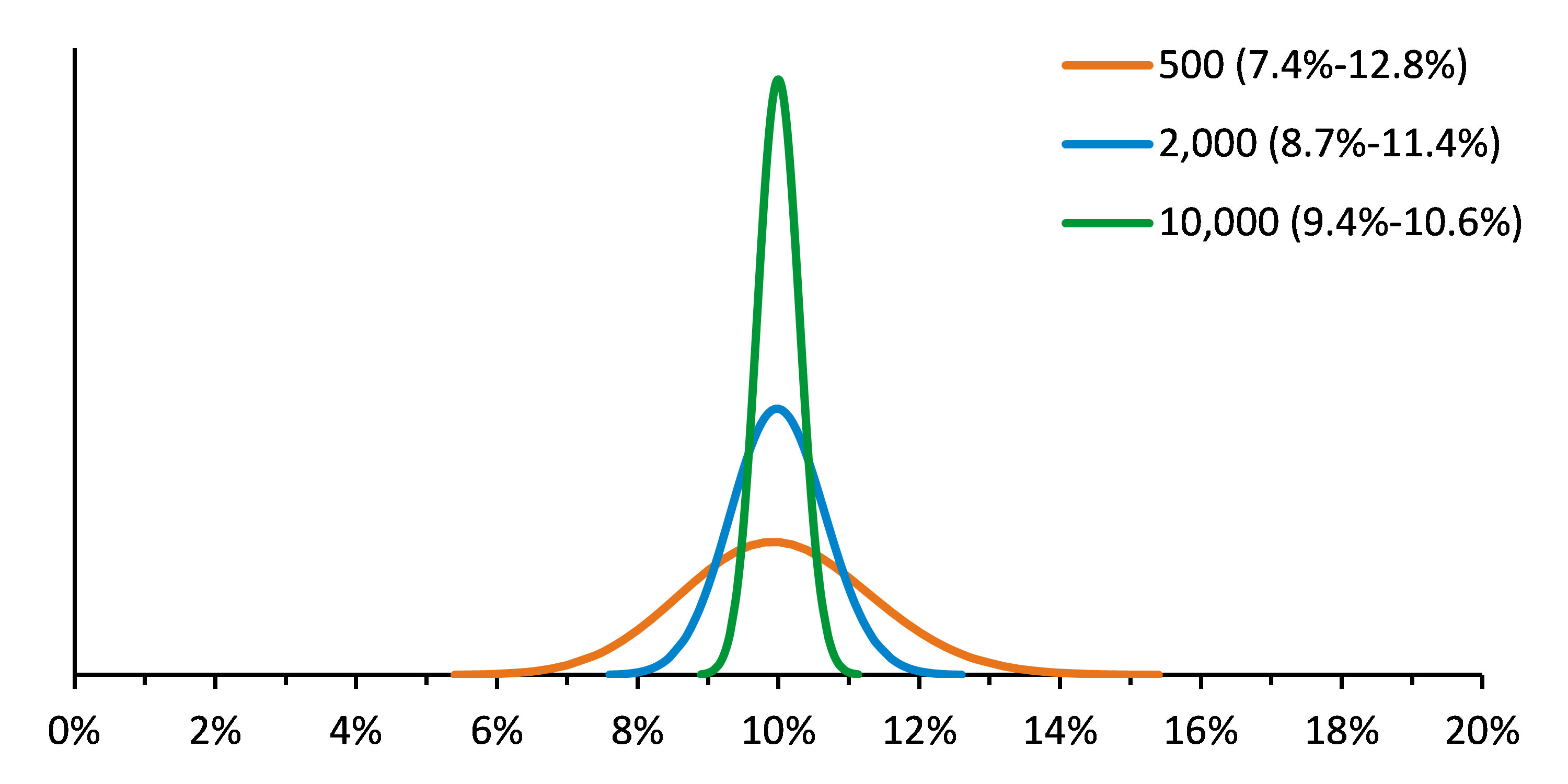
Sensitive ctDNA Assays are Required for Minimal Residual Cancer Detection
Posted by
Russell Garlick, PhD on May 29, 2018 12:00:00 AM
The 11th International Symposium on Minimal Residual Cancer was held this month and much of the conference was devoted to new minimally invasive methods for circulating tumor cell enrichment and or the analysis of circulating tumor DNA. Today’s clinical needs are to measure disease burden, track mutations over time, or to detect early resistance and all of these applications require extremely sensitive, robust assays.
0 Comments Click here to read/write comments