Choose your Article Focus | NGS | Molecular & Serology
Omo Clement, PhD
Recent Posts
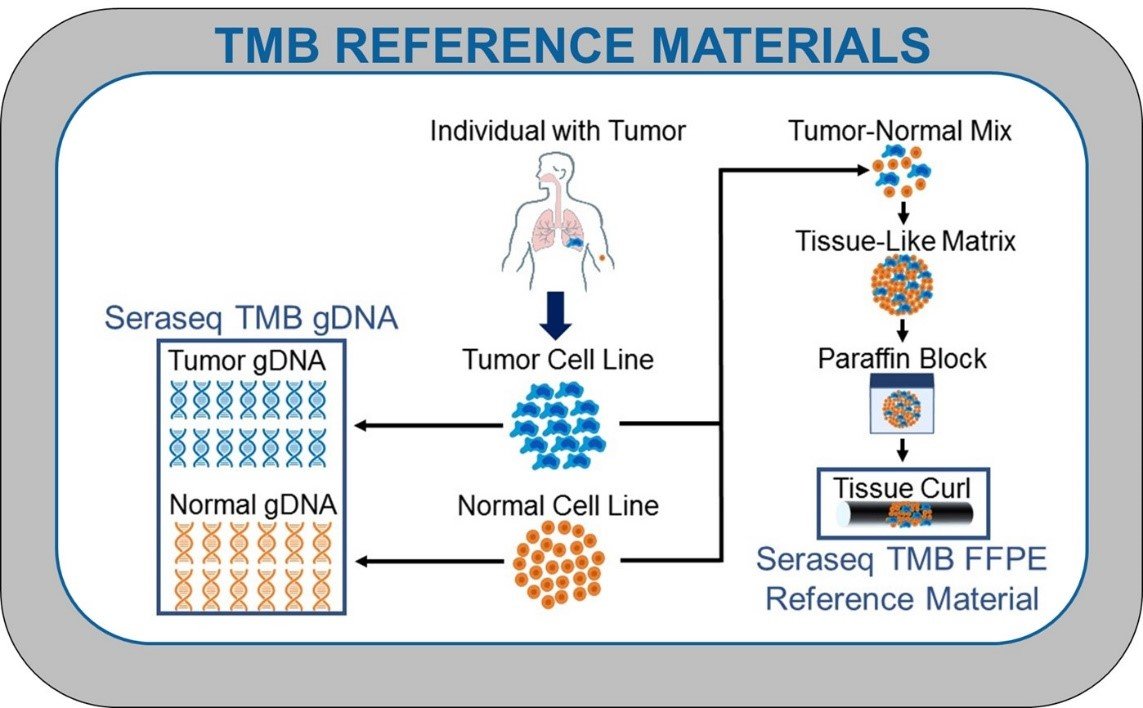
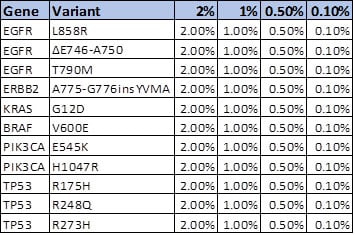
Advancing Immuno-Oncology Biomarker Validation with Industry’s First NGS-based TMB Reference Materials
Category: NGS, Immuno-Oncology
Posted by
Omo Clement, PhD on Sep 2, 2020 12:00:00 AM
Overview Tumor mutational burden (TMB) is a measurement of the number of mutations carried by a tumor cell genome. By comparing DNA sequences from a patient’s healthy tissue and tumor cells, and using a number of complex algorithms, scientists can determine the number of acquired somatic mutations present in tumors but not in normal tissues.1 NGS is the primary method employed to measure TMB, either through targeted panels or whole exome sequencing (WES);2 the latter is considered the gold-standard measurement today.
0 Comments Click here to read/write comments
Customer Data: Use of Seraseq ctDNA Reference Samples to Validate Detection of Low Frequency Variants in a cfDNA-based NGS Lung Cancer Panel
Category: AMP, SeraSeq, cfDNA, Lung Cancer, ctDNA
Posted by
Omo Clement, PhD on Feb 14, 2019 12:00:00 AM
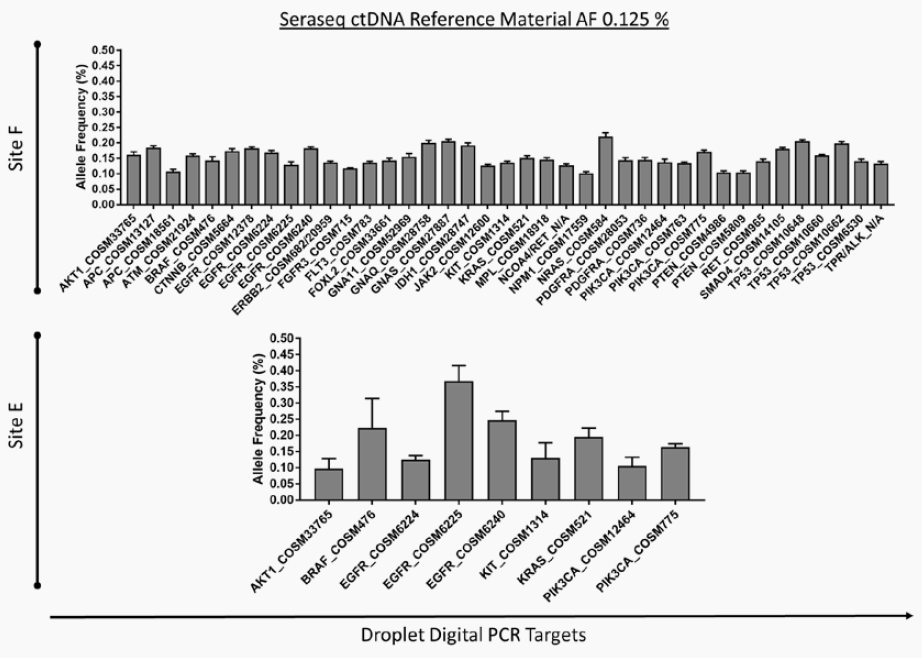
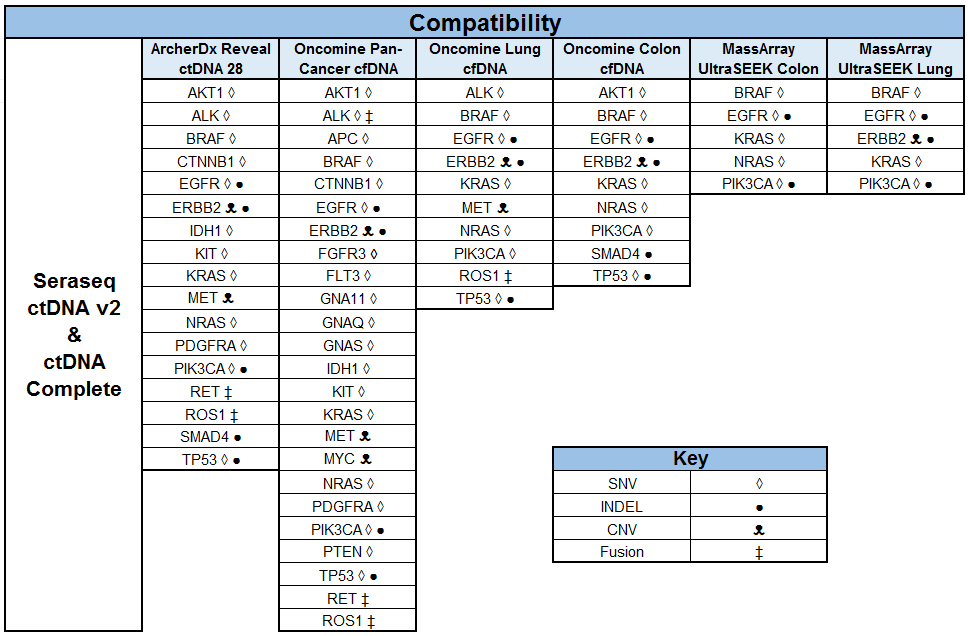
At the recently-concluded 2018 AMP Meeting, researchers at the New York Presbyterian Hospital (NYPH) and Weill Cornell Medical Center (WCMC) presented a poster1 on the validation of an Oncomine™ cell-free DNA Lung Assay using ctDNA NGS standards developed by SeraCare (Seraseq® ctDNA v2 Reference Materials),2
0 Comments Click here to read/write comments
NTRK Fusion Testing for New Therapies
Category: NTRK, cancer, RNA fusion
Posted by
Omo Clement, PhD on Feb 6, 2019 12:00:00 AM
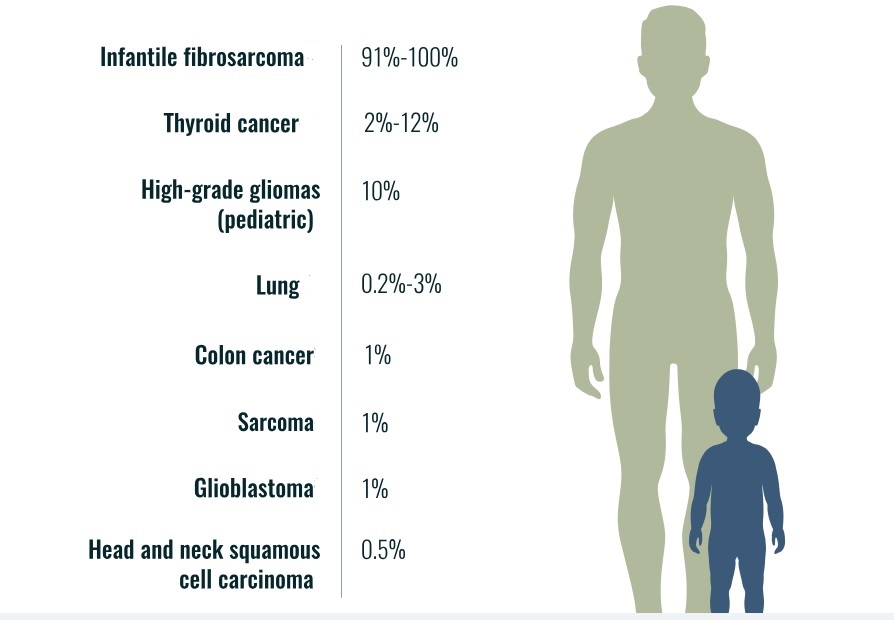
Neurotrophic tyrosine receptor kinases (NTRK) can become abnormally fused to other genes resulting in growth signals that can lead to cancer in many organs of the human body. TRK gene fusion-based cancers are rare but present in pediatric and adult cancers such as lung, thyroid, colon, etc. (see, e.g., Figure 1).
0 Comments Click here to read/write comments
Building and Implementing Liquid Biopsy Assays with the Industry’s Most Patient-Like Reference Materials
Category: SeraSeq, liquid biopsy, NGS, reference materials
Posted by
Omo Clement, PhD on Oct 24, 2018 12:00:00 AM
SeraCare’s clinical genomics technologies are developed to address challenges faced across the spectrum of NGS assays. From early development of assays – either IVD assay manufacturers or clinical labs building their own LDTs - there is a scarcity of characterized, complex, difficult variants to ensure the assay can robustly detect all the critical genomic variants in a patient sample. Using our highly characterized, reproducible, and GMP-grade NGS standards, laboratories have a wide range of analytical and clinical validation tools to deeply characterize assay performance such as LOD, linearity, specificity, sensitivity, and reproducibility.
0 Comments Click here to read/write comments
How A New Generation of ctDNA Reference Standards Are Enabling the Promise of Precision Medicine
Category: SeraSeq, liquid biopsy, NGS, cancer, ctDNA
Posted by
Omo Clement, PhD on Jun 14, 2018 12:00:00 AM
An important goal in cancer disease management is early detection. When detected early, disease progression can be significantly mitigated with a plethora of options (targeted therapy, chemotherapy, surgery, etc.) available to medical practitioners, to afford progression free survival and a higher quality of life. A great promise of liquid biopsies is the possibility of early detection of cancer long before clear evidence of lesions and tumor growth observable by imaging or other techniques.1 As proxy for solid tissue biopsies, plasma-based liquid biopsy application is rapidly gaining traction in cancer disease diagnosis, progression, monitoring, and in predicting resistance to treatment options.2
0 Comments Click here to read/write comments