Choose your Article Focus | NGS | Molecular & Serology
Sam Blier
Recent Posts
So Many Posters, So Little Time
Category: TMB, RNA fusion, ctDNA, AACR
Posted by
Sam Blier on Jun 6, 2019 12:00:00 AM
Cancer research is purposely methodical and measured. So – somewhat paradoxically – it can be difficult to keep up with the steady stream of discoveries in the literature and presented at conferences like AACR. As a developer and manufacturer of platform-agnostic NGS reference standards, we’re in a unique position to collaborate with cancer genomics assay developers, laboratories, pharmaceutical companies, and other organizations invested in more precise and robust cancer tests.
0 Comments Click here to read/write comments
Keys to Better Liquid Biopsy Assay Sensitivity – AMP Corporate Workshop Video
Category: AMP, liquid biopsy, NGS, ctDNA
Posted by
Sam Blier on Jan 24, 2019 12:00:00 AM
“So as everyone here is aware, I’m sure, detection of circulating tumor DNA is challenging. There’s very little of it, to start with.” Hardly a revolutionary statement by Tony Godfrey, PhD, (Associate Chair, Surgical Research and Associate Professor of Surgery, Boston University School of Medicine) but an important acknowledgement from a leading expert of the difficulty faced by laboratorians
0 Comments Click here to read/write comments
Workshop Video: Two Experts Take on Clinical Genomics QC and Standardization at AMP
Category: AMP, clinical genomics, NGS
Posted by
Sam Blier on Dec 18, 2018 12:00:00 AM
If you’ve attended the AMP Annual Meeting over the years or seen any of the headlines it generates, you know how next-generation sequencing-based assays are becoming indispensable diagnostic, prognostic, and predictive tools for a growing number of disease states. But just as important as the newest biomarker or latest chemistry – but seemingly less headline-worthy – are NGS quality control and standardization.
0 Comments Click here to read/write comments
Two Must-See Liquid Biopsy Poster Videos From AMP 2018
Category: AMP, SeraSeq, liquid biopsy, cfDNA, ctDNA
Posted by
Sam Blier on Dec 7, 2018 12:00:00 AM
Of the many fantastic posters presented at AMP’s Annual Meeting in San Antonio, two concerning NGS-based liquid biopsy assays stood out. Both presenters described how their organizations are working to reliably detect pathogenic variants at extremely low allele frequencies – efforts critical to the clinical adoption of NGS-based liquid biopsy assays.
0 Comments Click here to read/write comments
Expert opinions on LDT transparency and standardization
Category: clinical genomics, LDT
Posted by
Sam Blier on Oct 6, 2017 12:00:00 AM
Laboratory-developed tests (LDTs) have proliferated in the absence of clear guidelines and regulations. So how can laboratorians, physicians, and patients be assured of the quality of the diagnostic result? A panel of clinical genomics experts (Girish Putcha, MD, PhD, Director of Laboratory Science, Palmetto GBA; Roger Klein, MD, Principal, JD Consulting; Elaine Lyon, PhD, Medical Director, Molecular Genetics and Genomics, ARUP Laboratories; and Russell Garlick, PhD, CSO, SeraCare) delved into this topic during the audience Q&A session of a recent webinar hosted by GenomeWeb (you can download a full report on the entire series here).
0 Comments Click here to read/write comments
You can’t validate everything… can you?
Category: clinical genomics, QC data management
Posted by
Sam Blier on Sep 21, 2017 12:00:00 AM
“Is there a limit on the type of mutations we would need to validate given that the cost per validation is high?” This question was recently posted to a panel of clinical genomics experts that we convened for a webinar hosted by GenomeWeb (you can download a full report on the entire series here). Panelists Girish Putcha, MD, PhD, Director of Laboratory Science, Palmetto GBA; Roger Klein, MD, Principal, JD Consulting; and Elaine Lyon, PhD, Medical Director, Molecular Genetics and Genomics, ARUP Laboratories, weighed in with thoughtful responses to a query that’s on the minds of many clinical laboratorians.
0 Comments Click here to read/write comments
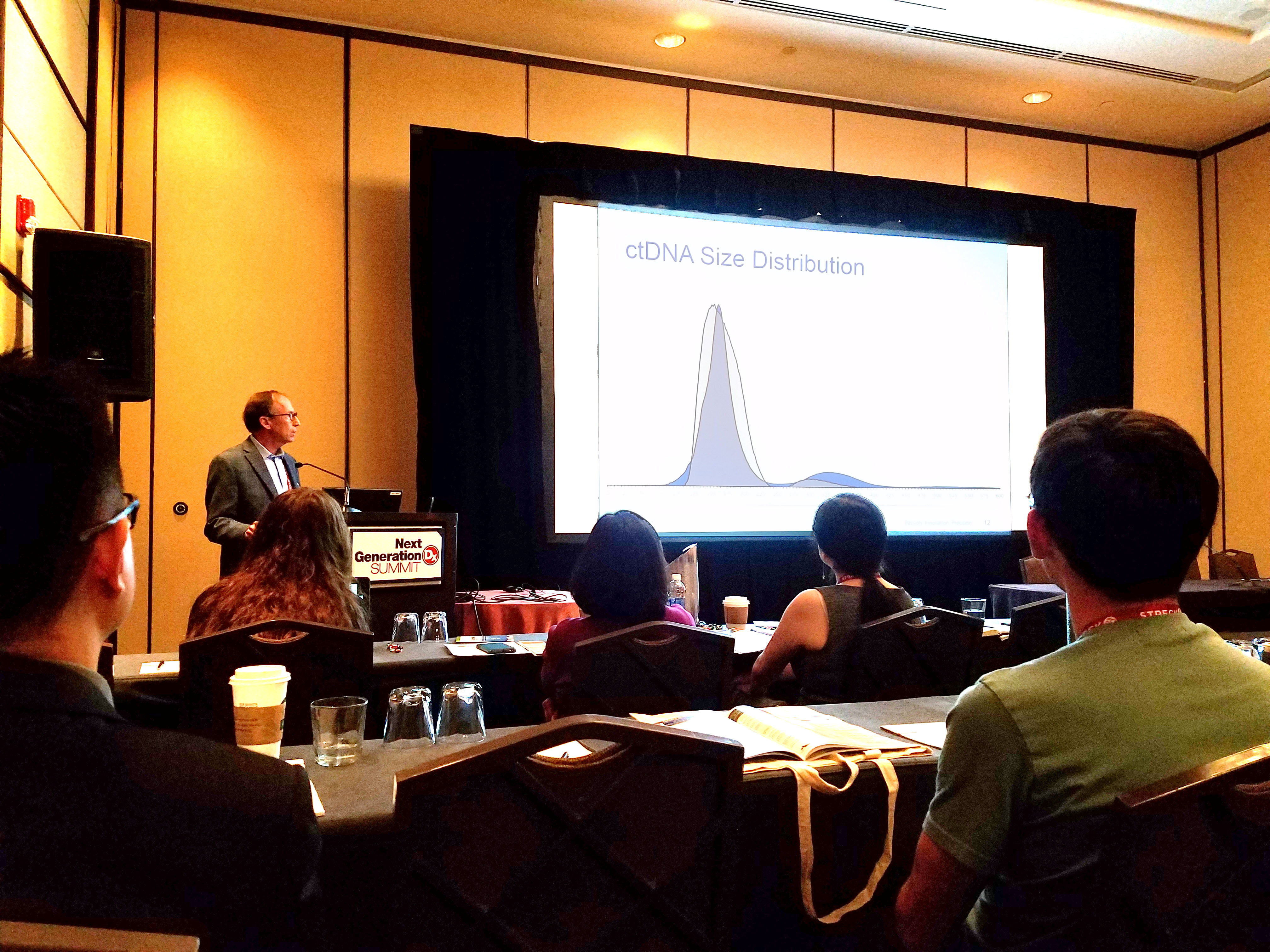
Accelerating Liquid Biopsy Assay Development
Category: liquid biopsy, Assay Development, ctDNA, reference materials
Posted by
Sam Blier on Sep 8, 2017 12:00:00 AM
At the 2017 Next Generation Dx Summit in Washington, DC, our CSO, Russell Garlick, PhD, presented a workshop on accelerating liquid biopsy assay development. He has worked closely with a variety of groups in the liquid biopsy space that are developing and validating circulating tumor DNA (ctDNA) assays. He highlighted some common challenges facing the field, and explained how SeraCare has been using these collaborations to develop QC tools specifically for ensuring the robustness of these cutting-edge tests.
0 Comments Click here to read/write comments