Choose your Article Focus | NGS | Molecular & Serology
Dale Yuzuki
Recent Posts
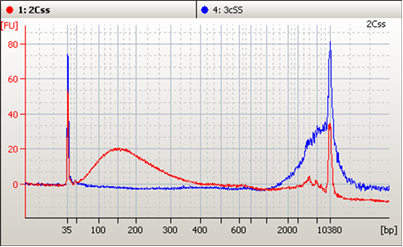
Three liquid biopsy problems solved by the most patient-like ctDNA reference materials
Category: cfDNA, ctDNA, reference materials
Posted by
Dale Yuzuki on Jul 24, 2017 12:00:00 AM
Clinical genomics laboratories are increasingly looking to liquid biopsy cancer assays to complement their current solid tumor assays. Compared to their solid tumor assay counterparts, circulating tumor DNA (ctDNA) assays offer a different set of challenges to consider for clinical labs. One of the most important of which, is to develop a set of reagents that are appropriately validated to determine the critical performance of the assay across many parameters. The ctDNA targets of liquid biopsy assays are typically at much lower allelic frequencies and require a robust and reproducibly designed assay to consistently detect these important variants.
0 Comments Click here to read/write comments
What specifically contributes to high levels of NGS accuracy?
Category: clinical genomics
Posted by
Dale Yuzuki on Jul 7, 2017 12:00:00 AM
Accuracy of NGS results is extremely critical for clinical genomic laboratories, when developing, validating or running an assay. At the 2017 Precision Medicine World Conference a panel discussion entitled ‘Achieving Accurate NGS Test Results’ featured Dr. Greg Tsongalis, Director of Clinical Genomics and Advanced Technology at Dartmouth Hitchcock Medical Center; Dr. Dara Aisner, Associate Professor of Pathology at University of Colorado Anschutz Medical Campus School of Medicine; and Dr. Russell Garlick, Chief Scientific Officer of SeraCare Life Sciences.
0 Comments Click here to read/write comments
Dr. Andrea Ferreira-Gonzalez on the Seven Benefits of Clinical Genomics Universal Standardization
Category: clinical genomics, NGS
Posted by
Dale Yuzuki on Jun 22, 2017 12:00:00 AM
As a 25-year veteran of clinical molecular diagnostics, Dr. Andrea Ferreira-Gonzalez has seen many changes in genetic technologies used in the testing laboratory. With the advent of personalized medicine and using multi-gene NGS panels as a laboratory-developed test, Dr. Ferreira-Gonzalez and other experts have agreed to lend their expertise to the design of SeraCare’s reference materials. She and other groups have participated in an interlaboratory test of standardized reference materials for detecting cancer somatic mutations, with results that will be published in the coming months.
0 Comments Click here to read/write comments
Dr. Andrea Ferreira-Gonzalez on the Seven Benefits of Clinical Genomics Universal Standardization
Category: clinical genomics, laboratory training, NGS, reference materials
Posted by
Dale Yuzuki on Jun 22, 2017 12:00:00 AM
As a 25-year veteran of clinical molecular diagnostics, Dr. Andrea Ferreira-Gonzalez has seen many changes in genetic technologies used in the testing laboratory. With the advent of personalized medicine and using multi-gene NGS panels as a laboratory-developed test, Dr. Ferreira-Gonzalez and other experts have agreed to lend their expertise to the design of SeraCare’s reference materials. She and other groups have participated in an interlaboratory test of standardized reference materials for detecting cancer somatic mutations, with results that will be published in the coming months.
0 Comments Click here to read/write comments
How Many Target Copies Are Present in Your Plasma DNA Sample?
Category: liquid biopsy, cancer, Assay Development, ctDNA
Posted by
Dale Yuzuki on Jun 15, 2017 12:00:00 AM
The field of circulating tumor DNA analysis (ctDNA, also sometimes called in a larger context “liquid biopsy”) holds great promise for monitoring response to cancer treatment, assisting therapeutic choice, monitoring recurrence, and for pre-cancer screening. As such there is a great amount of assay development and ongoing clinical trials; at ClinicalTrials.gov searching for the term "Circulating DNA" you can find over 180 open clinical trials for a wide range of tumor types and interventions.
0 Comments Click here to read/write comments
How to Validate Your Lab’s Clinical Genomics Tests Affordably … and Revalidate
Category: ngs validation, NGS
Posted by
Dale Yuzuki on May 25, 2017 12:00:00 AM
For clinical genomics testing laboratories, validation — and to a certain extent, revalidation — is a fact of life. It’s written into the CAP/CLIA regulations. The regulations say you must validate: Any new FDA-cleared test your lab introduces. Any modifications you make to an FDA-cleared test. Any laboratory-developed (LDT) test not subject to FDA regulation. In the case of the first one, validation means you can replicate the performance specifications determined by the manufacturer of the test. In the second two, however, validation involves determining the performance specifications yourself (in seven areas, as outlined by CLIA). How do you go about testing the accuracy of a test your lab purchased or determining the accuracy of a test your lab designed?
0 Comments Click here to read/write comments
Futureproof Your Clinical Genomics Lab’s Compliance: 3 Tips to Prepare for Potential Requirements
Category: FDA, clinical genomics
Posted by
Dale Yuzuki on May 18, 2017 12:00:00 AM
While clinical genomics labs (those running NGS-based laboratory developed tests) are not currently subject to FDA oversight, that doesn’t mean they won’t be someday. The question is, how soon will that day come and, when it does, will your lab be ready?
0 Comments Click here to read/write comments
3 Tips for Reducing the Costs of Running Your Clinical Genomics Lab (While Maintaining Accuracy and Efficiency)
Category: clinical genomics
Posted by
Dale Yuzuki on May 9, 2017 12:00:00 AM
When you’re in charge of quality control (QC) for a clinical genomics testing laboratory, you know that one word — quality — casts a wide net. For you, quality means (among many other things):
0 Comments Click here to read/write comments
Your Lab Is Growing; Here’s How to Train Your New Staff Quickly
Posted by
Dale Yuzuki on May 4, 2017 12:00:00 AM
Due to either staff turnover or increased testing demand, your clinical genomics laboratory is expanding. You need your new personnel trained and ready to perform at the same high level as the rest of your staff as soon as possible. You know that the best training programs are guided ones, with samples and workflows similar to those your new staff members will encounter on a day-to-day basis. Are your remnant patient samples up to the task?
0 Comments Click here to read/write comments
3 Root Causes of Downtime for Clinical Genomic Testing Labs (and How to Recover Fast)
Category: clinical genomics
Posted by
Dale Yuzuki on Apr 20, 2017 12:00:00 AM
Downtime can be devastating to a clinical testing laboratory. The timely return of test results is critical for effective patient care. Any delay can hurt your lab’s reputation and prompt your customers to seek testing services elsewhere. Unfortunately, any clinical testing laboratory using sophisticated next-generation sequencing or other genetic analysis technology will suffer downtime sooner or later. The question is, how fast can you recover? What Causes Downtime? There are myriad factors that could cause your laboratory to cease operations: Simple operator error - anything from a sample mix-up to a PCR contamination - can cause a downstream problem that can take days or weeks to identify and correct. Poorly-performing vendor supplied reagents, kits, instruments, or software. Operators Are Human; Mistakes Can and Do Happen As it makes its way through your lab, a patient sample interacts with a wide range of operators, materials, and instrumentation. Mistakes can creep in at any point along this process, including: Labeling and accessioning. The use of particular reagents. The setting of instrument parameters. The informatics pipeline. When an error does occur, it can take a long time and a lot of effort to determine root cause and take action to fix it. Without an appropriate reference standard, the problem becomes more complex, as the number of potential causes increases exponentially. Here is an example:
0 Comments Click here to read/write comments