This week is National Women’s Health week1 and is dedicated to women to focus on their health and take steps to improve it. As Women’s Health week traditionally starts on Mother’s Day, we tend to focus primarily on Reproductive Health but it is important to look at the complete picture as some women encounter various conditions and treatments prior to starting a family. Women’s health encompasses things like fertility, pregnancy, infections, cancer, but also many underlying health conditions such as hypertension, diabetes, cardiovascular or respiratory conditions.
Many of women’s health problems can be diagnosed by using advanced testing methods. As the technology develops and becomes more complex, ensuring the best performance of the tests is even more critical. With better testing, it is possible to improve outcomes for women around the world.
Overcoming the Struggles of Infertility
According to the CDC, infertility is a significant problem with about 10% of women in the US have difficulty getting pregnant2 but percentages of infertility vary globally, with the phenomenon not isolated to just developed countries.
There are many reasons for an increase in infertility, but a consistently cited cause is the increased age of first-time mothers3. Another factor affecting fertility are STIs. A global rise in infections, frequently undiagnosed and untreated, can lead to problems with conception4. The fact that many of the infections are asymptomatic makes them “invisible” to the patients and clinicians until they lead to other health problems including fertility issues. Additional causes of infertility can include hormonal imbalances due to polycystic ovarian syndrome (PCOS), ovarian insufficiency, inflammatory conditions, endometriosis or non-cancerous fibroids1.
Overcoming infertility became possible with the development of Assisted Reproductive Technology (ART). However, success of the implantation of the in vitro fertilized eggs remains variable. In order to improve implantation success of euploid embryos, a preimplantation screening or testing method known as PGS or PGT was implemented5,6. Preimplantation genetic testing for aneuploidies (PGT-A), is widely used and highly recommended to improve chances of pregnancy. There are multiple commercial tests available using single cell biopsy or embryo growth media.
While ART is primarily used for helping infertile couples, it can also be used to avoid transferring a genetic disease to a baby when both parents are the carriers of the disease-causing mutations. In this case, preimplantation genetic testing for monogenic disorders (PGT-M) is carried out on embryos to eliminate the possibility of having a baby with a serious and lethal condition.
The more we learn about new factors affecting fertility and expand our understanding of the effects, the more we will be able to successfully improve outcomes of infertility treatments and overcome the risk for serious disease.
Ensuring the Well Being of Mother and Baby
Expecting a baby is an exciting but also stressful time in a woman’s life. There are many changes happening to a woman’s body during this time and staying healthy is very important, as some maternal conditions, infections and genetic factors can affect the baby. Fortunately, there are many ways to screen pregnancies to ensure the wellbeing of the developing fetus, and the clinical practice for how this early this care can be provided is continually evolving.
Ultrasound technology has been in use since the 1970s (Figure 1) — the advantages of this non-invasive screening method are clear, and the method continues to improve. Detailed scans are currently available throughout the pregnancy and are part of the integrated screening. They are useful to ensure the wellbeing of baby and mother alike. In addition, studying pregnancy biomarkers enabled development of serum screening to identify potential fetal anomalies, such as trisomies or neural tube effects. These screening tests have been improving but they still have a relatively high false positive rate, at about 4-8% depending on the marker and test7.
A positive result in a screening test and any potential ultrasound anomalies generally means that an invasive diagnostic procedure is recommended. Two methods are available — amniocentesis and chorionic villus sampling (CVS). Amniocentesis tests the amniotic fluid around the fetus, while CVS tests placental cells and can pick local mosaicism rather than embryonic anomaly. Aside from the risks associated with potential miscarriage, these are not procedures, which appeal to women.
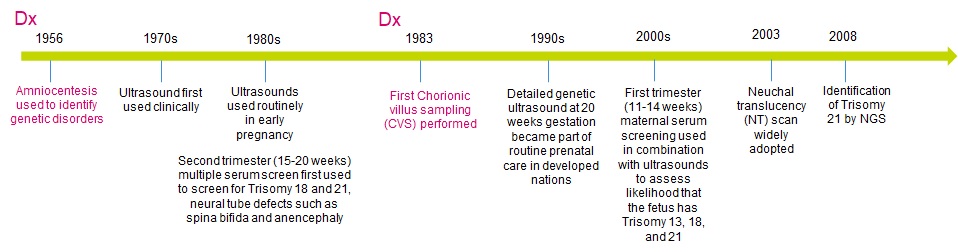
Figure 1. Changes in prenatal screening and diagnostic methods (Dx, in magenta)
These traditional screening methods rely on serum tests and ultrasounds and require multiple visits to the OB/GYN office. Under normal circumstances this is not a problem for most women. However, during an epidemiological emergency as we have right now with a SARS-CoV-2 pandemic, this is not the best solution8. In order to provide a high standard of care but also reduce office visits, some countries cancelled serum screens and replaced them with non-invasive prenatal testing (NIPT) or improved access to NIPT tests9.
NIPT tests are a screening method that rely on a blood draw from a pregnant woman and provide an assessment of the chromosomal content of the fetus. They are capable of identifying chromosomal aneuploidy and some microdeletions with far greater PPV than previous non-invasive test methods10. While the likelihood of aneuploidy increases with maternal age, microdeletion disorders occur at a fairly constant rate and are of concern for populations with lower maternal age. NIPT tests can also identify the sex of the fetus. The available methods are highly accurate, but they are not diagnostic and NIPT tests are not suitable for women expecting three or more babies. In order to confirm any anomalies, an invasive procedure, primarily amniocentesis, or occasionally CVS has to be performed. As such, there is a mild push to start referring to NIPT as non-invasive prenatal screening (NIPS) despite the term NIPT being well-established in the community.
NIPT tests are still developing and evolving, and there is increased adoption of these tests worldwide. This increased adoption is driving the need for patient-like materials to validate and/or verify the assays in new labs. In an increasingly competitive NIPT market, the use of independently verified reference materials is one of the ways to ensure high quality testing. In the coming years we may see an expansion of the screening technology beyond the NIPT, as tests are already available to screen for single gene disorders in a fetus11.
Improving Inherited Breast Cancer Screening
Breast cancer is the most common cancer in women globally12. However, only a small percentage of cases are inherited. The inherited version of breast cancer is driven primarily by mutations in BRCA1 and BRCA2 genes. Women carrying certain mutations in these two genes are over 65% more likely to develop breast cancer13. Screening for inherited BRCA1/2 mutations can be complicated as many variants are challenging in size or form (insertions or deletions as well as rare variants). Due to the relative biological complexity of functionally important BRCA1/2 mutations, test accuracy is critical to ensure correct reporting of mutations to both properly inform risk assessment and guide treatment.
Providing Confidence With More Accurate Results Through the Use of High-Quality NGS Reference Materials
The evolution of next generation sequencing and creation of multiple new assays to screen, test or diagnose is helping to improve the quality of care. It is now possible to use NGS to test for:
A change in how the diagnostic tests are done coupled with NGS tests becoming a new norm leads to an increased need for more genetics training for physicians. And this new education has already started with great resources provided, for example by Genomics England14.
These tests are also more complex in the way they are performed and interpreted. The proper performance of these tests is critical to ensure their value for the clinical application. One of the ways to improve the tests is by using high quality validation materials that can improve performance and standardize tests across the different methods and labs. At LGC SeraCare, we are proud to provide the labs with a number of products supporting development and performance of high-quality tests for women’s health. They include our reproductive health product line, germline cancer testing, breast cancer testing and infectious disease.
References:
- National Women’s Health Week. https://www.womenshealth.gov/nwhw/about. Last accessed 05/11/2020
- CDC. Infertility. https://www.cdc.gov/nchs/fastats/infertility.htm. Last accessed 05/11/2020
- Mascarenhas, M. N., Flaxman, S. R., Boerma, T., Vanderpoel, S. & Stevens, G. A. National, Regional, and Global Trends in Infertility Prevalence Since 1990: A Systematic Analysis of 277 Health Surveys. PLoS Med. 9, (2012). PMID: 23271957
- Tsevat, D. G., Wiesenfeld, H. C., Parks, C. & Peipert, J. F. Sexually transmitted diseases and infertility. American Journal of Obstetrics and Gynecology 216, 1-9 (2017). PMID: 28007229
- Chang, J., Boulet, S. L., Jeng, G., Flowers, L. & Kissin, D. M. Outcomes of in vitro fertilization with preimplantation genetic diagnosis: an analysis of the United States Assisted Reproductive Technology Surveillance Data, 2011–2012. Fertil. Steril. 105, 394–400 (2016). PMID: 26551441
- Fesahat, F., Montazeri, F. & Hoseini, S. Preimplantation genetic testing in assisted reproduction technology. J Gynecol Obs. Hum Reprod 49, 101723 (2020). PMID: 32113002
- Metcalfe, A., Hippman, C., Pastuck, M. & Johnson, J.-A. Beyond Trisomy 21: Additional Chromosomal Anomalies Detected through Routine Aneuploidy Screening. J. Clin. Med. 3, 388–415 (2014). PMID: 26237381
- Boelig, R. C., Saccone, G., Bellussi, F. & Berghella, V. MFM Guidance for COVID-19. Am. J. Obstet. Gynecol. MFM 100106 (2020) doi:10.1016/j.ajogmf.2020.100106. PMID: 32363335
- Health Insurance Fund to pay for NIPT testing for pregnant women. https://news.err.ee/1068966/health-insurance-fund-to-pay-for-nipt-testing-for-pregnant-women (2020). Last accessed 05/11/2020
- Porreco, R. P. et al. Evaluation of a novel screening method for fetal aneuploidy using cell-free DNA in maternal plasma. J. Med. Screen. 27, 1-8 (2019). PMID: 31510865
- Shaw, J., Scotchman, E., Chandler, N. & Chitty, L. Non-invasive prenatal testing for aneuploidy, copy number variants and single gene disorders. Reproduction (2020) doi:10.1530/rep-19-0591. PMID: 32130205
- WHO. Breast cancer: prevention and control. https://www.who.int/cancer/detection/breastcancer/en/index1.html. Last accessed 05/12/2020
- Kuchenbaecker, K. B. et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA - J. Am. Med. Assoc. 317, 2402-2416 (2017). PMID: 28632866
- NHS Health Education England. HEE Genomics Education Programme. https://www.genomicseducation.hee.nhs.uk/. Last accessed 05/12/2020




