Circulating tumor DNA detection by real-time quantitative PCR, digital PCR, or next-generation sequencing has garnered an abundance of scientific and commercial interest. We have written before about the critical need for standards and reference materials for this emerging translational oncology research and applied domains and we are pleased to report the launch of the Seraseq Circulating Tumor DNA Reference Materials.
A reproducible and consistent source of reference materials
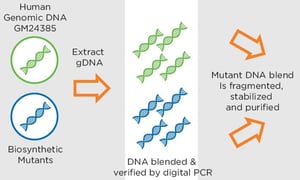
Manufactured through a similar process to our existing Seraseq Trisomy 21 Aneuploidy Reference Material, the Seraseq Circulating Tumor DNA-I Reference Material is comprised of a set of nine actionable ‘driver’ oncology mutations that are blended at a precise allele-frequency ratio to GM24385 human reference DNA, fragmented to approximately 170 base-pairs, stabilized via a patent-pending method, and mixed into a human plasma-like matrix. These products are offered at varying allele frequencies (5%, 1.2%, 0.6%, 0.1% and WT for ‘wild-type’ or 0%) as 5mL of material, with at least 60ng of extractable DNA per vial.
The Seraseq Circulating Tumor DNA-I Reference Material (AF5, AF1.2, AF0.6, AF0.1 or WT) serves as an ideal reference material to assist with control of the full-process, starting from nucleic acid extraction through assay (whether RT qPCR, digital PCR, or NGS-based) and analysis, to track and monitor your laboratory’s daily assay performance.
Purified material for assay development
The promise of circulating tumor nucleic acids, in a recent review from the Association for Molecular Pathology, states “more trials are required to validate the clinical utility of precise molecular markers for a variety of tumor types”. There are a number of assay development efforts underway, from both academic and commercial organizations. To assist with these efforts, SeraCare has also made purified nucleic acids available; the Seraseq Circulating Tumor DNA-I Mutation Mix (AF5-WT) is a set of five vials of purified, fragmented DNA for assay development and optimization.
More information about these products is available online, and feel free to contact us here or via the comments below.
.