Choose your Article Focus | NGS | Molecular & Serology
Mary-Claire King at the European Society for Human Genetics 2016
Category: cancer, Inherited Disease
Posted by
Dale Yuzuki on Jun 6, 2016 12:00:00 AM
Mary-Claire King is Professor of Genome Sciences and of Medicine (Medical Genetics) at the University of Washington (Seattle, WA), who first described a single gene on chromosome 17q21 (which she named BRCA1) being responsible for breast and ovarian cancer in many families. This work, published in Science in 1990, also revolutionized the study of many other common, single-gene diseases that are inherited in nature, and led to the discovery of BRCA2.
0 Comments Click here to read/write comments
SeraCare introduces AccuPlex™ Zika Reference Material
Category: zika virus, clinical genomics, Accuplex
Posted by
Dale Yuzuki on May 10, 2016 12:00:00 AM
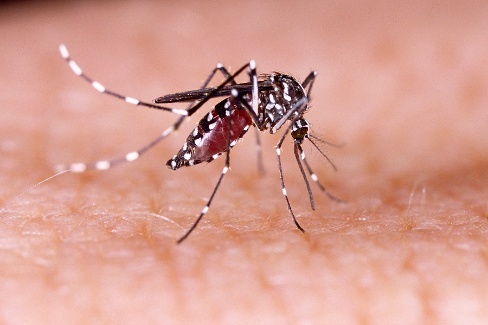
Guest author: Han Joo Lee, Ph.D., SeraCare Technical Product Manager A cursory look at recent Zika Virus outbreak news headlines is alarming enough: “Zika Virus birth defects may be the ‘Tip of the Iceberg’, experts say”; “Brain damage in Zika babies is far worse than doctors expected”; and “Zika mutates extremely quickly, which is why it’s so scary”. In addition, for the United States the estimated range maps of the Aedes aegypti and Aedes albopictus have been recently updated, widening the range previously reported to all but 10 states within the US.
0 Comments Click here to read/write comments
American Association for Cancer Research 2016 ctDNA highlights
Posted by
Dale Yuzuki on May 2, 2016 12:00:00 AM
At the American Association for Cancer Research conference recently held in New Orleans, LA (April 16 - April 20, 2016) one theme that generated considerable interest was circulating tumor DNA detection, sometimes called liquid biopsy (although that term also encompasses circulating tumor cells). A few of the ctDNA presentations are highlighted below.
0 Comments Click here to read/write comments
Previewing the American Association for Cancer Research 2016 Conference #AACR16
Category: clinical genomics, cancer, AACR
Posted by
Dale Yuzuki on Apr 16, 2016 12:00:00 AM
With over “Cancer Moonshot: A Call to Action” 20,000 scientists, the American Association for Cancer Research conference describes itself as a ‘must-attend event for cancer researchers and the broader cancer community’. One sign of the prominence of this event is the fact that US Vice President Joseph Biden will be delivering a special closing address, in connection with the recent announcement of the announced at the recent World Economic Forum.
0 Comments Click here to read/write comments
The FDA NGS-based Oncology Panels Workshop
Posted by
Yves Konigshofer, PhD on Apr 1, 2016 12:00:00 AM
The U.S. Food and Drug Administration on February 25, 2016 held an all-day public workshop entitled “Next Generation Sequencing-based Oncology Panels” at their White Oak campus in Silver Spring, Maryland. Companion diagnostics were a recurring theme of the initial FDA presentations at this Next Generation Sequencing-Based Oncology Panels Workshop and these important tests will be our focus here. The slides that were presented at the workshop are available (PDF) and are referenced below, and those presented by SeraCare’s CSO Russell Garlick start on page 196.
0 Comments Click here to read/write comments
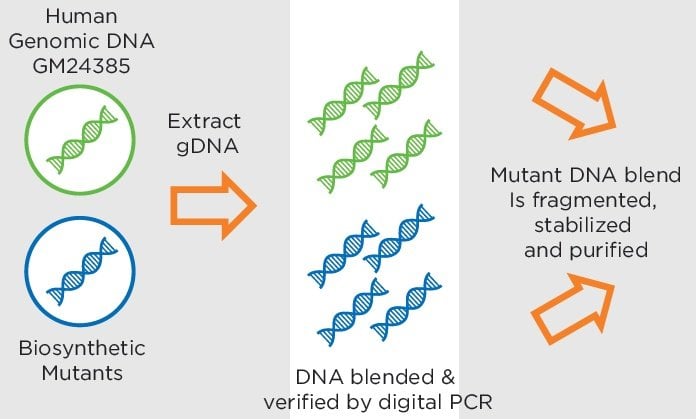
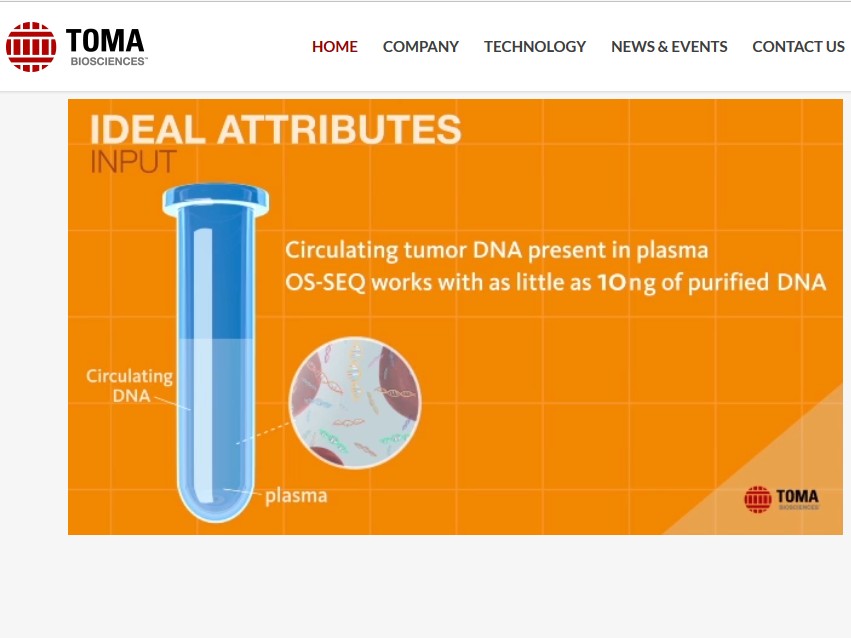
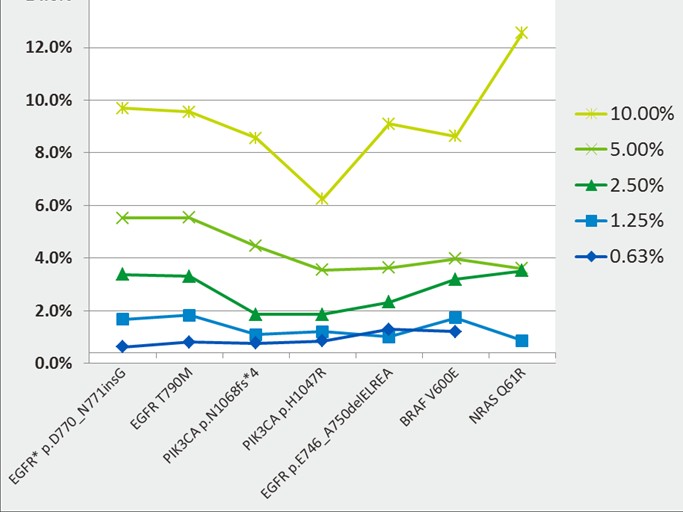
Circulating tumor DNA detection by real-time quantitative PCR, digital PCR, or next-generation sequencing has garnered an abundance of scientific and commercial interest. We have written before about the critical need for standards and reference materials for this emerging translational oncology research and applied domains and we are pleased to report the launch of the Seraseq Circulating Tumor DNA Reference Materials.
0 Comments Click here to read/write comments
Highlights from the 2016 Molecular Medicine Tri-Conference
Category: clinical genomics, cancer, Inherited Disease, ctDNA, reference materials
Posted by
Dale Yuzuki on Mar 22, 2016 12:00:00 AM
We are living through a time of rapid change in the clinical genetics laboratory, though at times it may appear that change doesn’t occur fast enough given the challenges within the existing healthcare system. At the recent Molecular Medicine Tri-Conference held in San Francisco March 7 through 11 2016, here are a few summary highlights of the conference.
0 Comments Click here to read/write comments
Molecular Tri-Conference Preview (San Francisco CA March 7-11 2016)
Posted by
Dale Yuzuki on Feb 29, 2016 12:00:00 AM
The 23rd annual Molecular Medicine Tri-Conference will be in San Francisco, California March 7 through 11, 2016, and will cover topics ranging from personalized diagnostics, circulating tumor cells, and clinical NGS diagnostics through integrated bioinformatics, frontiers in gene editing, cancer immunotherapy and molecular diagnostics for infectious disease.
0 Comments Click here to read/write comments
Oncology Regulatory Framework for NGS Webinar Friday Feb 26 2016
Posted by
Dale Yuzuki on Feb 22, 2016 12:00:00 AM
We are living at a time when genomic technologies (in particular next-generation sequencing) are revolutionizing the practice of medicine, in particular in the field of oncology. In addition, the US Food and Drug Administration has made it clear that these advanced technologies as they are applied to diagnostic testing need to come under regulatory oversight.
0 Comments Click here to read/write comments
Circulating Tumor DNA Poster at Keystone Symposium
Category: SeraSeq, clinical genomics, cancer, ctDNA, reference materials
Posted by
Dale Yuzuki on Feb 11, 2016 12:00:00 AM
The Keystone Symposia is an organization with 44 years of history on specialized topics across the fields of molecular and cellular biology. This week in Banff, Alberta, Canada is a Keystone Symposia conference called The Cancer Genome, along with a joint meeting on Genomics and Personalized Medicine. Their Twitter description (@KeystoneSymp) describes the Keystone organization as “A catalyst for accelerating life science discovery and connecting scientists within and across disciplines at symposia worldwide”.
0 Comments Click here to read/write comments