Choose your Article Focus | NGS | Molecular & Serology
Does synthetic DNA sequence behave the same as cell-line DNA?
Posted by
Yves Konigshofer, PhD on Feb 5, 2016 12:00:00 AM
A recurring question SeraCare has been asked is whether synthetic DNA sequences perform similarly to cell line-derived sequences. For example, it could be hypothesized that secondary structure and/or the GC content of the DNA around a particular amplicon might lead to a detection bias when only 400 bases on either side of the amplicon are present compared to when many kilobases are present.
0 Comments Click here to read/write comments
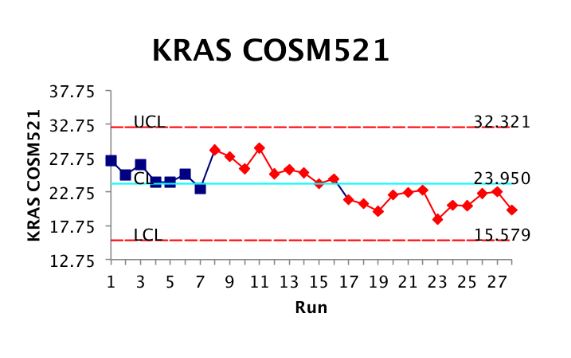
Analytical Validation using Biosynthetic Mutation Targets
Posted by
Dale Yuzuki on Jan 13, 2016 12:00:00 AM
The National Cancer Institute has started several significant clinical trials to confirm what is believed intuitively about precision medicine: that molecular characterization of tumors leading to personalized therapy may be more effective than standard cancer treatments. Announced in August of 2015, an NCI-sponsored effort called Molecular Analysis for Therapy Choice has 10 trial arms, with additional arms opening in April or May of 2016, with an ultimate goal of enrolling 3,000 patients. (More information about the MATCH trial can be found here.)
0 Comments Click here to read/write comments
LDT Oversight Counterpoint: Tempering FDA Arguments
Posted by
Russell Garlick, PhD on Jan 7, 2016 12:00:00 AM
As pointed out by my colleague Trevor Brown in his blog post “LDT Oversight: Why the FDA makes a point” (their case for increased oversight of the new wave of Laboratory Developed Tests [LDTs]), the LDT horse “left the barn” years ago so why is the FDA calling for a ‘round up’ today?
0 Comments Click here to read/write comments
Vaccines“Knowledge of science is one of the most beautiful things humans have to offer each other.” Alan Alda, Actor and Science Communicator, who has a namesake Alan Alda Center for Communicating Science, Stony Brook University.
0 Comments Click here to read/write comments
LDT Oversight: Why the FDA makes a point
Category: FDA, LDT, reference materials
Posted by
Trevor Brown on Dec 21, 2015 12:00:00 AM
Recently, the FDA upped the ante in the ongoing debate over its desire to regulate laboratory developed tests (LDTs) with the release of a report detailing the ‘real and potential harms to patients and to public health’ arising from LDTs. This debate has been heating up for several years now—not coincidentally with the emergence of precision medicine and the rapid adoption of data-intensive tools such as Next Generation Sequencing (NGS) and the growing pipelines of targeted therapeutics. One might argue that the horse has already left the barn and the FDA are trying to corral it back in.
0 Comments Click here to read/write comments
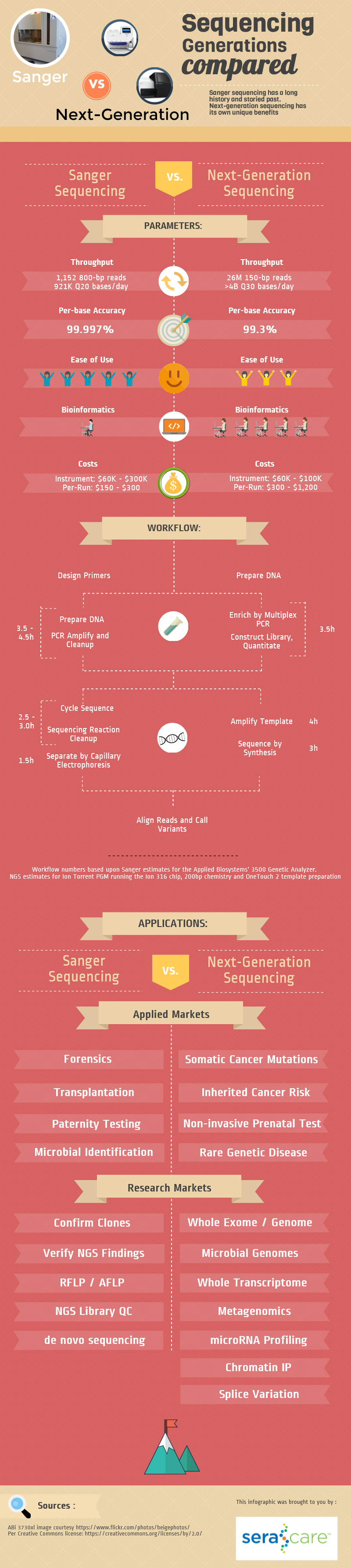
Sanger versus Next-Generation Sequencing Infographic
Category: SeraSeq, NGS, reference materials
Posted by
Dale Yuzuki on Dec 16, 2015 12:00:00 AM
It is hard to believe that next-generation sequencing has only been around for a little over ten years, but has had a profound impact on many frontiers of basic and applied genetics. To contrast the first-generation (Sanger sequencing by capillary electrophoresis) and the next-generation sequencing (NGS) approaches, we provide you with this infographic.
0 Comments Click here to read/write comments
The current state of non-invasive prenatal testing
Category: NIPT
Posted by
Yves Konigshofer, PhD on Dec 9, 2015 12:00:00 AM
Sometimes a single remark can effectively summarize a very complex problem. While the November 12, 2015 FDA Public Workshop on “Standard Based Approach to Analytical Performance Evaluation of Next Generation Sequencing In Vitro Diagnostic Tests” focused primarily on tests that look for germline and somatic variants, a comment made by Jared Maguire – Counsyl’s Director of Computational Biology – about 1 hour 14 minutes into the workshop explained a lot about the current state of non-invasive prenatal testing (NIPT) and what patients, physicians and insurers are experiencing; more on that later.
0 Comments Click here to read/write comments
Video Interview: Developing Aneuploidy Reference Materials
Category: SeraSeq, clinical genomics, NIPT, reference materials
Posted by
Dale Yuzuki on Nov 24, 2015 12:00:00 AM
0 Comments Click here to read/write comments
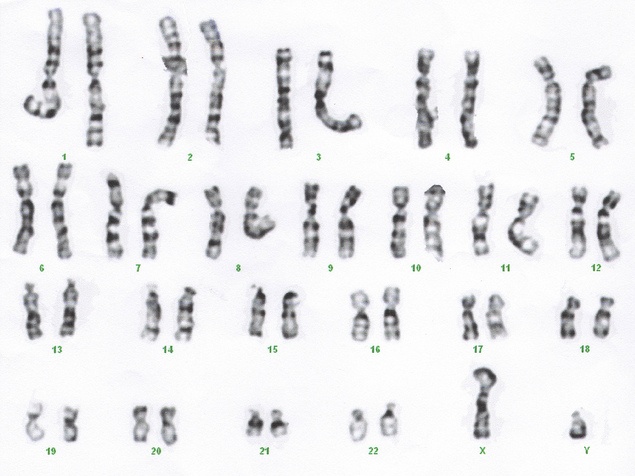
What is Non-Invasive Prenatal Testing (NIPT)?
Category: NIPT
Posted by
Dale Yuzuki on Nov 17, 2015 12:00:00 AM
Fetal aneuploidy affects about 9 in 1,000 live births. The definition of aneuploidy is an abnormal number of chromosomes ; with 23 pairs of chromosomes in humans, 46 is the normal number, while aneuploidy individuals will have 45 or 47. In trisomy, there is one additional chromosome, typically chr21, 18 or 13 (it is not a coincidence that these are the smallest chromosomes in humans). Historically, the invasive methods amniocentesis and chorionic villus sampling (CVS) were used with risk to the pregnancy, with about a 1% chance of miscarriage due to the procedure. Non-invasive methods based upon ultrasound and serum biomarkers are useful screening tests, but were of limited reliability as they were indirect measures of chromosomal abnormalities1.Photograph courtesy of Flickr user Can H.
0 Comments Click here to read/write comments
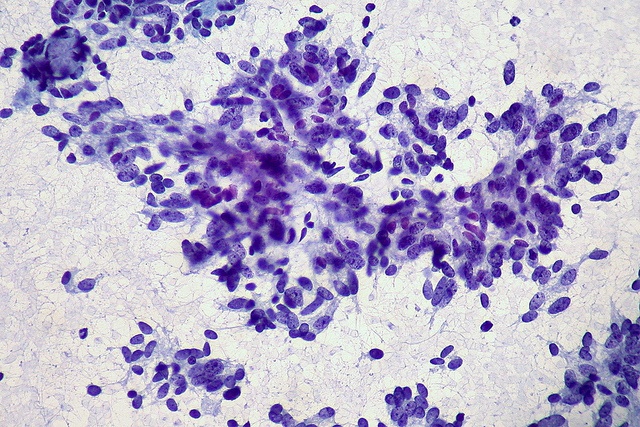
Association for Molecular Pathology 2015 Conference Highlights
Category: AMP, clinical genomics, cancer
Posted by
Dale Yuzuki on Nov 13, 2015 12:00:00 AM
Micrograph of Lung Carcinoma Fine Needle Aspirate courtesy of Ed Uthman via Flickr This year's Association for Molecular Pathology conference was held in Austin Texas November 4-7 2015 had the theme of 'Realizing the Dream of Precision Medicine'. Here are a few of the presentations that stood out as outstanding, and the conference program indicates where the field of molecular pathology currently places its emphasis (which is primarily oncology) and where it may be headed in the coming years (including rare genetic disorders, non-invasive prenatal testing, and even a plenary on the human microbiome).
0 Comments Click here to read/write comments