Choose your Article Focus | NGS | Molecular & Serology
Precision Medicine and Clinical Labs: AACR Dinner Seminar Recap
Category: clinical genomics, cfDNA, AACR
Posted by
Trevor Brown on Apr 15, 2019 12:00:00 AM
One of the core aims of precision medicine is to provide a more tailored approach to disease diagnosis, therapy selection, and patient monitoring to improve the overall quality of life for patients with disease. Indeed, this aim has been at the heart of the high interest and study of the potential of liquid biopsies to improve patient care in earlier detection of cancer, treatment, and surveillance.
0 Comments Click here to read/write comments
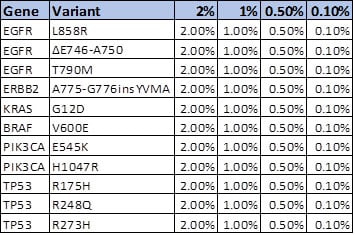
Customer Data: Use of Seraseq ctDNA Reference Samples to Validate Detection of Low Frequency Variants in a cfDNA-based NGS Lung Cancer Panel
Category: AMP, SeraSeq, cfDNA, Lung Cancer, ctDNA
Posted by
Omo Clement, PhD on Feb 14, 2019 12:00:00 AM
At the recently-concluded 2018 AMP Meeting, researchers at the New York Presbyterian Hospital (NYPH) and Weill Cornell Medical Center (WCMC) presented a poster1 on the validation of an Oncomine™ cell-free DNA Lung Assay using ctDNA NGS standards developed by SeraCare (Seraseq® ctDNA v2 Reference Materials),2
0 Comments Click here to read/write comments
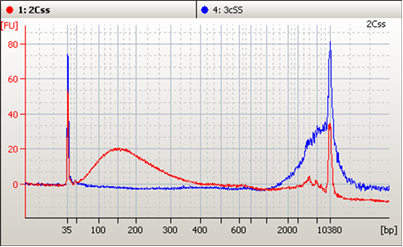
Two Must-See Liquid Biopsy Poster Videos From AMP 2018
Category: AMP, SeraSeq, liquid biopsy, cfDNA, ctDNA
Posted by
Sam Blier on Dec 7, 2018 12:00:00 AM
Of the many fantastic posters presented at AMP’s Annual Meeting in San Antonio, two concerning NGS-based liquid biopsy assays stood out. Both presenters described how their organizations are working to reliably detect pathogenic variants at extremely low allele frequencies – efforts critical to the clinical adoption of NGS-based liquid biopsy assays.
0 Comments Click here to read/write comments
Three liquid biopsy problems solved by the most patient-like ctDNA reference materials
Category: cfDNA, ctDNA, reference materials
Posted by
Dale Yuzuki on Jul 24, 2017 12:00:00 AM
Clinical genomics laboratories are increasingly looking to liquid biopsy cancer assays to complement their current solid tumor assays. Compared to their solid tumor assay counterparts, circulating tumor DNA (ctDNA) assays offer a different set of challenges to consider for clinical labs. One of the most important of which, is to develop a set of reagents that are appropriately validated to determine the critical performance of the assay across many parameters. The ctDNA targets of liquid biopsy assays are typically at much lower allelic frequencies and require a robust and reproducibly designed assay to consistently detect these important variants.
0 Comments Click here to read/write comments