Choose your Article Focus | NGS | Molecular & Serology
Insights on the Methylated DNA Webinar - Part 2
Category: ccfDNA, cfDNA, ctDNA, methylated, Methylated DNA
Posted by
Krystyna Nahlik, PhD on Jan 26, 2023 9:08:26 AM
DNA methylation is an epigenetic mechanism modulating the function and expression of genes. DNA methylation regulates gene expression by recruiting proteins involved in gene repression or by inhibiting the binding of transcription factor(s) to DNA. Changes in DNA methylation are associated with numerous diseases and abnormal DNA methylation has been implicated as one of the mechanisms driving tumor onset, development, progression and recurrence. Identification of tumor-associated methylation patterns is of great diagnostic potential, especially for early detection of disease. In Part 2 of this two-part blog series, the article continues to review the methylated DNA biomarkers in plasma webinar. As a reminder, Part 1 covered the main themes discussed by our invited webinar speakers - click here to read that article. To conclude the recent webinar hosted by LGC Clinical Diagnostics and GenomeWeb, Dr. Yves Konigshofer talked about levels of evidence required for clinical diagnostics, from analytical validity through clinical validity and clinical utility. Reference materials can help prove analytical validity and assess the impact of pre-analytical factors. Methylation reference materials that he is currently developing at LGC CDx address the analytical validity of 5’methylcytosine measurements in cfDNA.
0 Comments Click here to read/write comments
Insights on Methylated DNA Biomarkers Webinar - Part 1
Category: ctDNA, Methylated DNA
Posted by
Krystyna Nahlik, PhD on Jan 6, 2023 12:03:07 PM
DNA methylation is an epigenetic mechanism modulating the function and expression of genes. DNA methylation regulates gene expression by recruiting proteins involved in gene repression or by inhibiting the binding of transcription factor(s) to DNA. Changes in DNA methylation are associated with numerous diseases and abnormal DNA methylation has been implicated as one of the mechanisms driving tumor onset, development, progression and recurrence. Identification of tumor-associated methylation patterns is of great diagnostic potential, especially for early detection of disease. Recently LGC Clinical Diagnostics had the pleasure to host a webinar, bringing together a panel of academic, clinical, and industry experts in the field of methylated DNA testing, who discussed the promises and challenges of early cancer screening and recent technological advances. It was a great opportunity to hear from the pioneer of prenatal cfDNA testing, Dennis Lo, MD, PhD, and an expert in computational cancer genomics analysis, Jimmy Lin, MD, PhD, MHS, as well as Yves Konigshofer, PhD, the head of technology development at LGC Clinical Diagnostics.
0 Comments Click here to read/write comments
Development of a Novel Reference Material for MRD Monitoring Assays
Category: MRD, ccfDNA, cfDNA, Minimal Residual Disease, NGS, ctDNA, reference materials
Posted by
Yves Konigshofer, PhD on Aug 17, 2021 12:00:00 AM
Authors: Yves Konigshofer, PhD; Andrew Anfora, PhD; Omo Clement, PhD; and Krystyna Nahlik, PhD. LGC Clinical Diagnostics. Introduction Liquid biopsy methods developed for circulating tumor DNA (ctDNA) analysis in solid tumors are transforming clinical practice, allowing for non-invasive detection and assessment of earlier stages of disease, monitoring for resistance to therapy, and post-treatment monitoring for minimal residual disease (MRD) and recurrence of cancer. The presence of minimal residual disease may be prognostic in that is has been found to correlate with worse patient outcomes, so early and accurate measurement is crucial. ctDNA-based assays allow for the detection of MRD at earlier time points than standard clinical and imaging surveillance, and could allow for treatment modification based on real-time assessment of the tumor genomic landscape.
0 Comments Click here to read/write comments
Webinar on Genomic Testing to Support New Therapies for Advanced Cancer — Insights and Takeaways
Category: ccfDNA, cfDNA, NGS, Genomic Testing, ctDNA, reference materials
Posted by
Russell Garlick, PhD on Aug 11, 2021 12:00:00 AM
This is Part 1 of a 2-part blog reviewing the Genomic Testing webinar and panel discussion featuring Dara Aisner, MD PhD, George Green, PhD and Greg Tsongalis, PhD. With so much rich information, we will be posting two blogs. Part 1 will cover select important themes discussed by each speaker, and Part 2 will review the audience Q&A. Recently, I had the pleasure of participating in a webinar co-sponsored by LGC SeraCare and GenomeWeb. The topic was “Genomic Testing to Support New Therapies for Advanced Cancer”.
0 Comments Click here to read/write comments
The Promise of Liquid Biopsy: A Q&A with Dr. Claudia Vollbrecht
Category: ccfDNA, cfDNA, NGS, ctDNA, reference materials
Posted by
Andrew Anfora, PhD on Mar 24, 2021 12:00:00 AM
This is Part 3 in a 3-part Q&A blog series with a panel of liquid biopsy experts addressing many of the issues faced in developing and deploying NGS-based liquid biopsy assays for clinical applications in oncology. At a 2020 liquid biopsy webinar, Dr. Vollbrecht shared a molecular pathologist’s perspective on the current state of liquid biopsy. Laboratory processing and analysis of cfDNA samples is a multi-step process that requires a high degree of precision to achieve consistent results. Her presentation focused on pre-analytics variables, which are often left out of discussions and tend to focus on biochemical manipulation of isolated nucleic acids. Seemingly simple factors at the point of sample collection such as problems with blood test tube filling, storage and labelling are able to affect the cfDNA stability, abundance, and confound the reliability of final interpretation. Variation in sample treatment during laboratory processing, including but not limited to, cfDNA quantification and QC methodology are also amongst the challenges for liquid biopsy.
0 Comments Click here to read/write comments
The Promise of Liquid Biopsy: A Q&A with Professor Ed Schuuring
Category: ccfDNA, cfDNA, NGS, ctDNA, reference materials
Posted by
Krystyna Nahlik, PhD on Mar 10, 2021 12:00:00 AM
This is Part 2 in a 3-part Q&A with a panel of liquid biopsy experts addressing many of the issues faced in developing and deploying NGS-based liquid biopsy assays for clinical applications in oncology. At a 2020 liquid biopsy webinar, Professor Schuuring discussed the plethora of options available to detect low copy number mutations in plasma cfDNA of lung cancer patients. His research laboratory combines NGS, ddPCR, qPCR and mass spectrometry approaches to address three main applications: (1) primary diagnosis by detection of predictive mutations, (2) monitoring of treatment response based on changes in plasma mutant levels, and (3) detection of therapy resistance mechanisms.
0 Comments Click here to read/write comments
The Promise of Liquid Biopsy: A Q&A with Professor Sandi Deans
Category: ccfDNA, cfDNA, NGS, ctDNA, reference materials
Posted by
Krystyna Nahlik, PhD on Mar 4, 2021 12:00:00 AM
This is Part 1 in a 3- series deep-dive Q&A with expert panelists addressing many of the issues faced in developing and deploying NGS-based liquid biopsy assays for clinical applications in oncology. At the 2020 liquid biopsy webinar, Professor Sandi Deans highlighted a recent EQA scheme aimed at evaluating the standard of cfDNA testing in NSCLS and CRC patients. It was driven by demand from participants themselves, as well as pharmaceutical companies, IVD manufacturers and IQNPath (International Quality Network for Pathology).
0 Comments Click here to read/write comments
The Promise of Liquid Biopsy for Cancer Diagnostics and Therapeutic Monitoring: Are We There Yet?
Category: ccfDNA, cfDNA, NGS, ctDNA, reference materials
Posted by
Krystyna Nahlik, PhD on Feb 25, 2021 12:00:00 AM
This is the introduction to a 3-part Q&A with a panel of liquid biopsy experts addressing some of the issues faced in developing and deploying NGS-based liquid biopsy assays for clinical applications in oncology. In this 3-part blog series, we will share highlights from a recent Liquid Biopsy Expert Panel webinar sponsored by LGC Seracare and facilitated by GenomeWeb, which brought together academic research and clinical experts in liquid biopsy technologies to discuss the benefits, shortcomings, challenges and recommendations for liquid biopsy adoption in the context of cancer disease management. The webinar drew a lot of interest and sparked in-depth questions from attendees, which required a post webinar follow-up response from all 3 panelists.
0 Comments Click here to read/write comments
How to resolve the challenges of MRD?
Category: MRD, cfDNA, NGS, ctDNA, reference materials
Posted by
Yves Konigshofer, PhD on Oct 7, 2020 12:00:00 AM
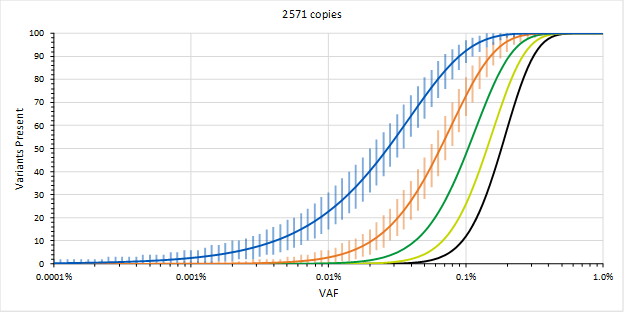
This is part 2 of 2 of the MRD blog post. (Click here for part 1). In this section, we will discuss how to overcome some of the most common challenges of MRD testing. Overcoming the Challenges In order to mitigate sequencing errors, methods using Molecular Barcodes (MBCs), Unique Molecular Identifiers (UMIs), etc. (which are essentially all the same) may be used, where each starting molecule is sequenced many times. The MBCs are then used to generate consensus sequences from sequences that were likely obtained from the same starting molecule. The assumption is that errors appear due to somewhat stochastic processes and that the consensus sequences will likely be correct. This requires many observations of the same starting molecule, so it will be recommended to generate 10-fold more sequences than there are molecules. Therefore, with 8,000 genomic equivalents, we might want to target a sequencing depth of 80,000. This is a reason why using 10-fold more input ccfDNA may not necessarily be a good thing (in addition to having to obtain a 10-fold larger liquid biopsy) since we may have to increase sequencing depth accordingly to 800,000, which could increase the cost of sequencing 10-fold, which could reduce the likelihood for payment and running the assay profitably.
0 Comments Click here to read/write comments
So, you want to monitor Measurable Residual Disease? What are the challenges?
Category: MRD, cfDNA, Minimal Residual Disease, NGS, ctDNA, reference materials
Posted by
Yves Konigshofer, PhD on Oct 1, 2020 12:00:00 AM
Part 1 of 2 Background Measurable Residual Disease (MRD) monitoring – for purposes of this blog – will be the act of looking for somatic variants in a liquid biopsy sample by analyzing circulating cell-free DNA (ccfDNA). This is done to monitor the disappearance of a metastatic solid tumor during treatment and to follow any future reemergence of that cancer. Analyzing ccfDNA assumes that circulating tumor DNA (ctDNA) will be present, and the median ctDNA frequency in patient samples across cancers seems to be around 0.5 to 1 %. Thus, the median variant allele frequencies (VAFs) of the somatic variants will start around this range, and the goal of MRD monitoring is to be able to detect them at much lower VAFs. This can be challenging and if we are going to design an assay for MRD monitoring, then we need to be aware of them and overcome them.
0 Comments Click here to read/write comments