Choose your Article Focus | NGS | Molecular & Serology
How to resolve the challenges of MRD?
Category: MRD, cfDNA, NGS, ctDNA, reference materials
Posted by
Yves Konigshofer, PhD on Oct 7, 2020 12:00:00 AM
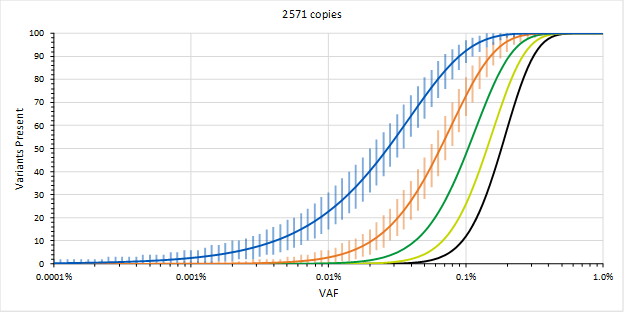
This is part 2 of 2 of the MRD blog post. (Click here for part 1). In this section, we will discuss how to overcome some of the most common challenges of MRD testing. Overcoming the Challenges In order to mitigate sequencing errors, methods using Molecular Barcodes (MBCs), Unique Molecular Identifiers (UMIs), etc. (which are essentially all the same) may be used, where each starting molecule is sequenced many times. The MBCs are then used to generate consensus sequences from sequences that were likely obtained from the same starting molecule. The assumption is that errors appear due to somewhat stochastic processes and that the consensus sequences will likely be correct. This requires many observations of the same starting molecule, so it will be recommended to generate 10-fold more sequences than there are molecules. Therefore, with 8,000 genomic equivalents, we might want to target a sequencing depth of 80,000. This is a reason why using 10-fold more input ccfDNA may not necessarily be a good thing (in addition to having to obtain a 10-fold larger liquid biopsy) since we may have to increase sequencing depth accordingly to 800,000, which could increase the cost of sequencing 10-fold, which could reduce the likelihood for payment and running the assay profitably.
0 Comments Click here to read/write comments
So, you want to monitor Measurable Residual Disease? What are the challenges?
Category: MRD, cfDNA, Minimal Residual Disease, NGS, ctDNA, reference materials
Posted by
Yves Konigshofer, PhD on Oct 1, 2020 12:00:00 AM
Part 1 of 2 Background Measurable Residual Disease (MRD) monitoring – for purposes of this blog – will be the act of looking for somatic variants in a liquid biopsy sample by analyzing circulating cell-free DNA (ccfDNA). This is done to monitor the disappearance of a metastatic solid tumor during treatment and to follow any future reemergence of that cancer. Analyzing ccfDNA assumes that circulating tumor DNA (ctDNA) will be present, and the median ctDNA frequency in patient samples across cancers seems to be around 0.5 to 1 %. Thus, the median variant allele frequencies (VAFs) of the somatic variants will start around this range, and the goal of MRD monitoring is to be able to detect them at much lower VAFs. This can be challenging and if we are going to design an assay for MRD monitoring, then we need to be aware of them and overcome them.
0 Comments Click here to read/write comments
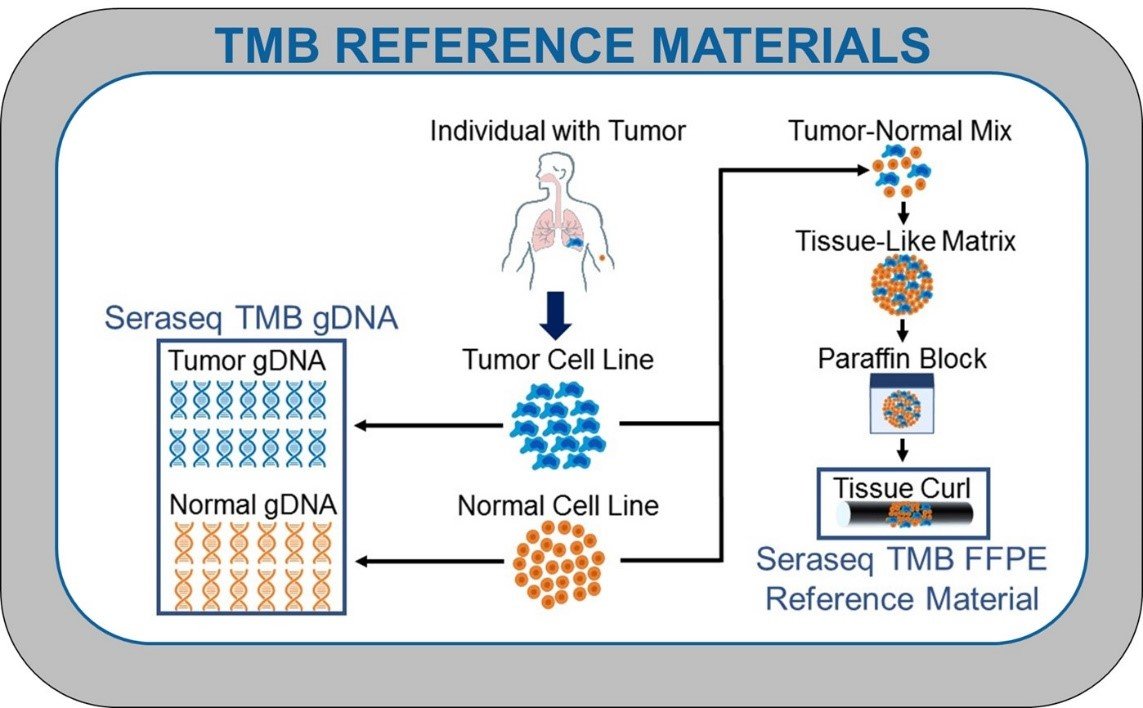
Advancing Immuno-Oncology Biomarker Validation with Industry’s First NGS-based TMB Reference Materials
Category: NGS, Immuno-Oncology
Posted by
Omo Clement, PhD on Sep 2, 2020 12:00:00 AM
Overview Tumor mutational burden (TMB) is a measurement of the number of mutations carried by a tumor cell genome. By comparing DNA sequences from a patient’s healthy tissue and tumor cells, and using a number of complex algorithms, scientists can determine the number of acquired somatic mutations present in tumors but not in normal tissues.1 NGS is the primary method employed to measure TMB, either through targeted panels or whole exome sequencing (WES);2 the latter is considered the gold-standard measurement today.
0 Comments Click here to read/write comments
Highlights from the ESHG and ESHRE 2020 Virtual Conferences
Category: Reproductive Health, PGT, Women's health, NIPT, Non-invasive Prenatal Testing, NGS, Preimplantation genetic testing
Posted by
Agnes Caruso,PhD on Aug 20, 2020 12:00:00 AM
The plans for this year’s NIPT and PGT conferences, like many others, were quickly derailed by the COVID-19 pandemic. The ESHG and ESHRE Annual Meetings were moved to virtual space and even though the format was a new experience, the science did not disappoint. Here we will share some of the highlights from the sessions.
0 Comments Click here to read/write comments
Design and Development of Compromised FFPE Reference Materials
Category: NGS
Posted by
Dana Ruminski Lowe, Ph.D. on Aug 11, 2020 12:00:00 AM
Tumor profiling assay workflows start from a tissue biopsy sample that is formalin fixed and paraffin embedded (FFPE), a process that introduces various kinds of damage in the nucleic acids in the tissue specimen. Reference materials that closely mimic the damage profile of patient FFPE samples are lacking. Depurination, depyrimidination, deamination, oxidation, nicks, and double strand breaks may be found in DNA extracted from FFPE tissue, despite the use of extraction kits that attempt to repair some of this damage. We have developed a formalin-damaged, multiplexed biosynthetic reference material, Seraseq® Compromised FFPE Tumor DNA, as well as a companion wild-type (WT) material, Seraseq Compromised FFPE WT, to mimic the damage found in patient samples that can be used to assess the entire tumor profiling workflow. We compared the performance of these reference materials in downstream assays to that of FFPE material with minimal DNA damage such as the Seraseq FFPE WT (DNA/RNA) RM product.
0 Comments Click here to read/write comments
Supporting High-Quality Testing for Women’s Health
Category: Reproductive Health, HIV, NIPT, Non-invasive Prenatal Testing, Breast Cancer
Posted by
Agnes Caruso,PhD on May 15, 2020 12:00:00 AM
This week is National Women’s Health week1 and is dedicated to women to focus on their health and take steps to improve it. As Women’s Health week traditionally starts on Mother’s Day, we tend to focus primarily on Reproductive Health but it is important to look at the complete picture as some women encounter various conditions and treatments prior to starting a family. Women’s health encompasses things like fertility, pregnancy, infections, cancer, but also many underlying health conditions such as hypertension, diabetes, cardiovascular or respiratory conditions. Many of women’s health problems can be diagnosed by using advanced testing methods. As the technology develops and becomes more complex, ensuring the best performance of the tests is even more critical. With better testing, it is possible to improve outcomes for women around the world.
0 Comments Click here to read/write comments
Do COVID-19 infections affect NIPT?
Category: COVID-19, NIPT, Non-invasive Prenatal Testing, NGS, SARS-CoV-2
Posted by
Prof. Joris Vermeesch, PhD on Mar 26, 2020 12:00:00 AM
We would like to present a guest blog from Prof. Joris Vermeesch, Ph.D. This blog originally posted on the Noninvasive Prenatal Testing Online Resource Page on the 17th March summarizes our current understanding of any possible interference of SARS-CoV-2 infection on the outcomes of NIPT testing.
0 Comments Click here to read/write comments
Assessing RNA Extraction with FFPE Fusion RNA Reference Materials
Category: NGS, RNA fusion, reference materials
Posted by
Dan Brudzewsky on Mar 11, 2020 12:00:00 AM
This is a third blog in a series on RNA fusions, this time focusing on how the FFPE Fusion RNA materials are used as RNA extraction controls.
0 Comments Click here to read/write comments
Diagnostic Testing Schemes for NTRK Cancers: All Roads Lead to NGS
Category: SeraSeq, NTRK, NGS, RNA fusion
Posted by
Russell Garlick, PhD on Feb 12, 2020 12:00:00 AM
New treatment options for cancer patients with neurotrophic tyrosine kinase (NTRK) rearrangements are a tremendous success, demonstrating first-hand the importance of precision diagnostics. The FDA granted accelerated approval of Bayer's VITRAKVI (larotrectinib) for adult and pediatric solid tumors containing NTRK fusions1. The approval was one of the first tissue agnostic approvals based on genotyping results, and included patients with unresectable or metastatic cancer. For 12 cancer types, the overall response rate was 75% with 22% complete response and 53% partial response. In addition to VITRAKVI, Genentech's entrectinib has a "breakthrough" status and is being evaluated for the potential treatment of advanced or metastatic tumors that harbor NTRK or ROS1 gene rearrangements.
0 Comments Click here to read/write comments
Evolution of non-invasive prenatal testing (NIPT)
Category: New Reference Material, trisomy, Reproductive Health, NIPT, Non-invasive Prenatal Testing, NGS, #Quality, reference materials
Posted by
Agnes Caruso,PhD on Feb 5, 2020 12:00:00 AM
Prenatal screening for aneuploidy has changed dramatically since the 1970s. Non-invasive methods developed in the 1980s and 1990s, combined measurements of maternal serum analytes and ultrasonography. The problem with those methods was not just a high false-negative rate of 12% to 23%, a high positive rate of 5% and a poor sensitivity, ranging from 50% to 95% 1. Uncertain results frequently led to invasive procedures such as amniocentesis or chorionic villi sampling to perform karyotyping on fetal samples. Both of those procedures carry a risk of miscarriage.
0 Comments Click here to read/write comments