Choose your Article Focus | NGS | Molecular & Serology
Multi-Lab Study of Fusion RNA Reference Standards for Targeted NGS
Category: NTRK, NGS, RNA fusion, reference materials, AACR
Posted by
Andrew Anfora, PhD on Jan 28, 2020 12:00:00 AM
Sourcing assay validation samples as positive run controls or workflow controls in targeted NGS RNA fusion assays remains a challenge today. This is further exacerbated with clinical labs looking to provide validated NGS assays for patient stratification in a host of new drugs in clinical trials or newly approved targeting fusion genes, such as NTRK genes (Larotrectinib, Loxo/Bayer) and Entrectinib (Genentech/Roche) for rare cancers in adult and pediatric patients, and RET (Loxo/Lilly) for lung cancer. SeraCare produces several RNA fusion reference materials. This article describes the development and multi-laboratory evaluation of a pan-cancer multiplexed Fusion RNA reference standard for analysis of clinically relevant fusion genes in solid tumors. The evaluation was conducted at 5 different laboratories on different NGS platforms (amplicon- and hybridization capture-based) as well as at different RNA inputs within a platform. Results highlight the utility of this Fusion RNA reference material to support clinical NGS assays as positive controls in solid tumor cancer patient stratification for many of these fusion-based targeted therapies.
0 Comments Click here to read/write comments
A Multi-Lab Study of Fusion RNA Reference Standards Using Amplicon- and Hybrid Capture-based Targeted NGS Panels
Category: NGS, Assay Development, RNA fusion
Posted by
Andrew Anfora, PhD on Jan 28, 2020 12:00:00 AM
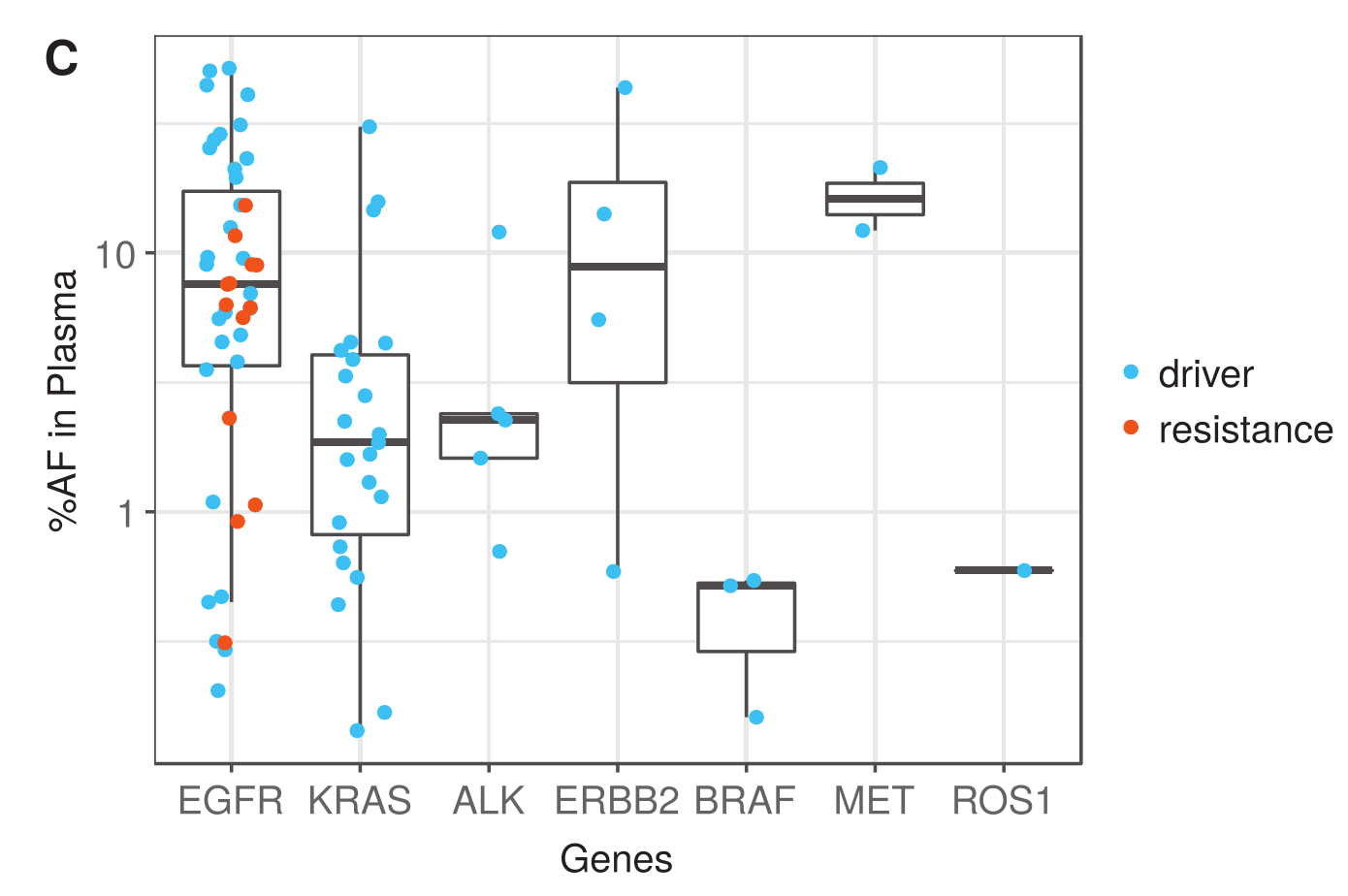
Introduction Sourcing assay validation samples as positive run controls or workflow controls in targeted NGS RNA fusion assays remains a challenge today. This is further exacerbated with clinical labs looking to provide validated NGS assays for patient stratification in a host of new drugs in clinical trials or newly approved targeting fusion genes, such as NTRK genes (Larotrectinib, Loxo/Bayer) and Entrectinib (Genetech/Roche) for rare cancers in adult and pediatric patients, and RET (Loxo/Lilly) for lung cancer. SeraCare produces several RNA fusion reference materials. This article describes the development and multi-laboratory evaluation of a pan-cancer multiplexed Fusion RNA reference standard for analysis of clinically relevant fusion genes in solid tumors. The evaluation was conducted at 5 different laboratories on different NGS platforms (amplicon- and hybridization capture-based) as well as at different RNA inputs within a platform. Results highlight the utility of this Fusion RNA reference material to support clinical NGS assays as positive controls in solid tumor cancer patient stratification for many of these fusion-based targeted therapies. Data Discussion Fusion RNA Reference Standard Development 18 clinically actionable biosynthetic fusion genes were transcribed and blended with reference cell line human cell line (GM24385, from GIAB), The RNA fusion constructs in the Fusion RNA reference material were blended at ~1500 copies per uL, and measured by digital droplet PCR (ddPCR) measurements as well as by an orthogonal targeted NGS assay (Archer FusionPlex Solid Tumor and MiSeq v2 300 cycle kit). Results are shown in Figure 1 below. Figure 1: Concentration (blue) and number of unique start sites (green) measured for each fusion RNA in the fusion reference material as determined by RT-dPCR and targeted NGS panel, respectively. Data represent average of three replicate measurements. As shown in Figure 1, all 18 gene fusions in the Fusion RNA reference material were detected on the Archer ST assay, at different levels of abundance. Approximately 50% of the variants showed similar relative abundance between ddPCR and NGS measurements. ~25% of the variants showed an apparent increase in relative abundance as measured by ddPCR, and the remaining ~25% of the gene fusions showed an increase in relative abundance as measured by NGS. A multi-laboratory evaluation of the Fusion RNA reference material was conducted at 5 laboratories (Site A – E, see Table 1). However, this article will focus on evaluations involving the Archer FusionPlex Solid Tumor and custom Solid Tumor-based targeted NGS panels. For discussion of all data generated by all laboratories listed in Table 1, check out our poster presentation from AACR 2018. Table 1: Assay, input, and analysis used at Site A – E. Figure 2 demonstrates the reproducibility of the Archer FusionPlex Solid Tumor assay across multiple sites and operators from the highly multiplexed contrived Fusion RNA reference material. The discordant data points are likely due to a combination of different operators, use of different analysis software versions, and differences in replicate measurements between sites. To account for the multitude of panel-based assays, one laboratory used a custom panel assay to evaluate the RNA fusion reference material. Beta test Site D used the fusion reference material with a customized Archer FusionPlex assay as shown in Figure 3. The functionality of the customized panel was clearly demonstrated, in addition, a limit of detection study was conducted with the abundant reference material, with most fusions detected at all tested input amounts.[1] [1] For another RNA fusion limit of detection study see our AACR 2019 poster. Summary SeraCare has developed a reference material that contains 18 clinically relevant RNA fusions. The RNA fusion reference material was evaluated at 6 different laboratories. Results of these analysis highlight the robustness of the Seraseq Fusion RNA reference materials in multi-lab concordance studies as well as a tool uniquely suited for limit of detection (LoD) studies. The RNA fusion reference material is also used in analytical validation of targeted NGS panels, and as an objective reference standard for inter-laboratory comparison of NGS assay performance. A similar multi-lab evaluation of the FFPE Fusion RNA reference materials will be published shortly.
0 Comments Click here to read/write comments
What is Non-Invasive Prenatal Testing (NIPT)?
Category: New Reference Material, trisomy, Reproductive Health, NIPT, #Quality, reference materials
Posted by
SeraCare Team on Jan 20, 2020 12:00:00 AM
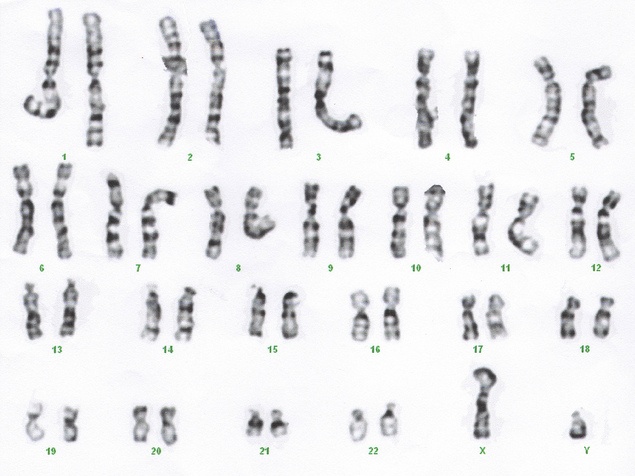
Fetal aneuploidy affects about 9 in 1,000 live births. The definition of aneuploidy is an abnormal number of chromosomes ; with 23 pairs of chromosomes in humans, 46 is the normal number, while aneuploidy individuals will have 45 or 47. In trisomy, there is one additional chromosome, typically chr21, 18 or 13 (it is not a coincidence that these are the smallest chromosomes in humans). Historically, the invasive methods amniocentesis and chorionic villus sampling (CVS) were used with risk to the pregnancy, with about a 1% chance of miscarriage due to the procedure. Non-invasive methods based upon ultrasound and serum biomarkers are useful screening tests, but were of limited reliability as they were indirect measures of chromosomal abnormalities1. Photograph courtesy of Flickr user Can H.
0 Comments Click here to read/write comments
So Many Posters, So Little Time
Category: TMB, RNA fusion, ctDNA, AACR
Posted by
Sam Blier on Jun 6, 2019 12:00:00 AM
Cancer research is purposely methodical and measured. So – somewhat paradoxically – it can be difficult to keep up with the steady stream of discoveries in the literature and presented at conferences like AACR. As a developer and manufacturer of platform-agnostic NGS reference standards, we’re in a unique position to collaborate with cancer genomics assay developers, laboratories, pharmaceutical companies, and other organizations invested in more precise and robust cancer tests.
0 Comments Click here to read/write comments
Newly Published Multi-Laboratory Study Provides Utility and Validation of the Use of ctDNA Reference Standards
Posted by
Russell Garlick, PhD on May 30, 2019 12:00:00 AM
I am pleased to share findings from a newly published peer-reviewed study with foundational circulating tumor DNA (ctDNA) pre-analytical and analytical testing in multiple technologies and assay chemistries. The study, “Multi-laboratory Assessment of a New Reference Material for Quality Assurance of Cell-Free Tumor DNA Measurements,” was just published in The Journal of Molecular Diagnostics (He, Stein et al. 2019).
0 Comments Click here to read/write comments
Making the Shift from Technological Innovation to Operational Excellence: Delivering on the Promise Of Next-Generation Sequencing for Personalized Medicine
Category: clinical genomics, NGS
Posted by
Peter Duncan on Apr 22, 2019 12:00:00 AM
As originally seen in The Journal of Precision Medicine March 2019. Targeted therapies and now recently, immunotherapies, have demonstrated great promise towards increasing response rates, as well as duration of response for cancer patients. This is often achieved by understanding biomarkers associated with therapeutic response and then stratifying patients accordingly.
0 Comments Click here to read/write comments
Precision Medicine and Clinical Labs: AACR Dinner Seminar Recap
Category: clinical genomics, cfDNA, AACR
Posted by
Trevor Brown on Apr 15, 2019 12:00:00 AM
One of the core aims of precision medicine is to provide a more tailored approach to disease diagnosis, therapy selection, and patient monitoring to improve the overall quality of life for patients with disease. Indeed, this aim has been at the heart of the high interest and study of the potential of liquid biopsies to improve patient care in earlier detection of cancer, treatment, and surveillance.
0 Comments Click here to read/write comments
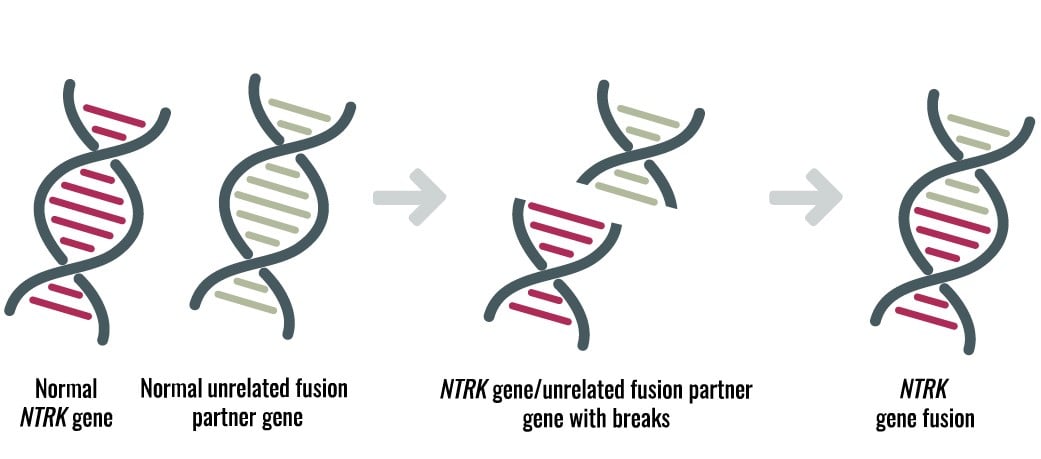
Presenting NTRK Reference Materials for Global Assay Standardization at AACR 2019
Category: SeraSeq, NTRK, RNA fusion, AACR
Posted by
Catherine Huang, PhD on Apr 8, 2019 12:00:00 AM
On the last morning of AACR 2019, I had the privilege of presenting a poster together with my colleague, Sebastian Bender from Bayer AG, in Berlin. Because of this, I didn’t have a chance to attend any talks, but I still wanted to finish out my blog series with highlights from each day of the conference.
0 Comments Click here to read/write comments
AACR 2019 Day Three: Understanding T-cell Therapy for Unique Cancer Mutations
Posted by
Catherine Huang, PhD on Apr 4, 2019 12:00:00 AM
My third day at the AACR Annual meeting was a day of phenomenal presentations. I am struggling to choose just one to tell you about because I attended multiple inspiring, thought-provoking, and even entertaining talks today. I decided to report on the plenary presentation by Steven A. Rosenberg entitled “T-cell therapy targeting unique cancer mutations” because I think this story has the most potential to positively impact patient outcomes.
0 Comments Click here to read/write comments
AACR 2019 Day Two: Learning About the Microbiome in Immuno-Oncology
Posted by
Catherine Huang, PhD on Apr 3, 2019 12:00:00 AM
At the AACR Annual Meeting, I was most excited to attend the major symposium entitled “The Microbiome as an Orchestrator of Immunity and Cancer Immunotherapy,” which featured three highly informative talks. First, Gregory Sonnenberg from Weill Cornell Medicine gave an overview of how the microbiome contributes to immune homeostasis in the intestine.
0 Comments Click here to read/write comments





-blocks-the-binding-site-of-PD-1-(red)-on-a-T-cell-to-avoid-the-down-regulation-of-the-immune-system-by-cancer-cells_-1053-1.jpeg)

