Choose your Article Focus | NGS | Molecular & Serology
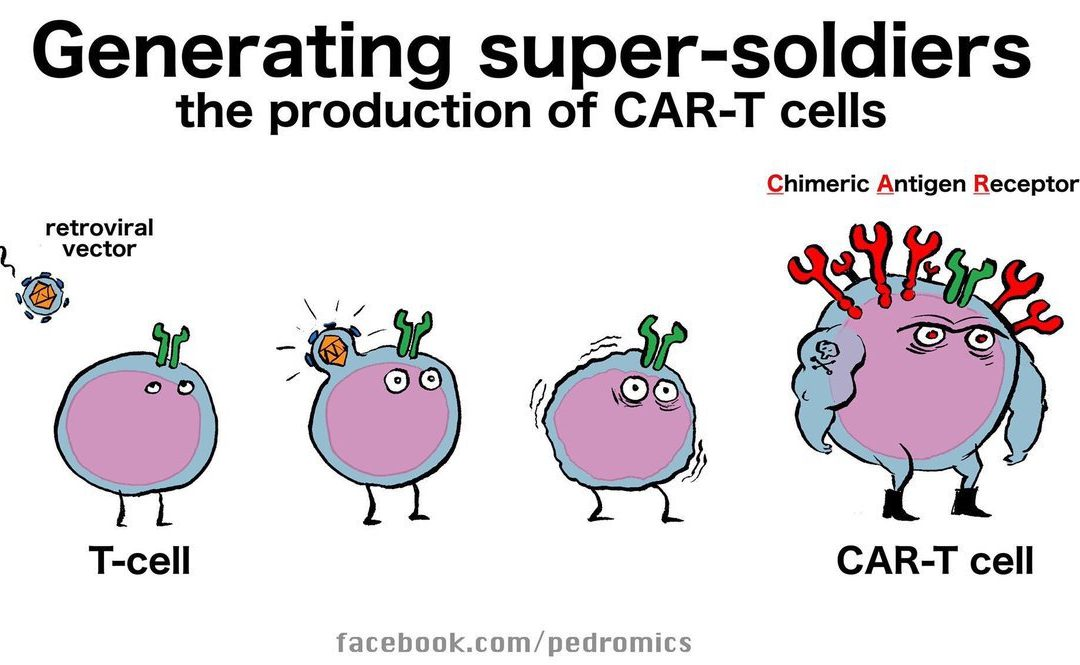
AACR 2019 Day One Highlights: Next-Generation Car T Cells
Posted by
Catherine Huang, PhD on Apr 2, 2019 12:00:00 AM
I’m excited to be at the Annual AACR meeting at the Georgia World Congress Center in Atlanta, GA. AACR is my favorite scientific meeting because each year I am inspired by the remarkable research presented and leave the conference feeling I’ve learned an incredible amount in just a few days.
0 Comments Click here to read/write comments
New Podcast: Target the Tumor Mutational Burden and Pursue Harmonization
Posted by
Melinda Fayette on Mar 20, 2019 12:00:00 AM
Friends of Cancer Research aims to better understand the impact of assay variation on clinical outcomes, align standards, and define best practices for tumor mutational burden assessment. Harmonization of methods to quantify TMB will facilitate robust biomarker development and optimize clinical utilization and treatment decision-making.
0 Comments Click here to read/write comments
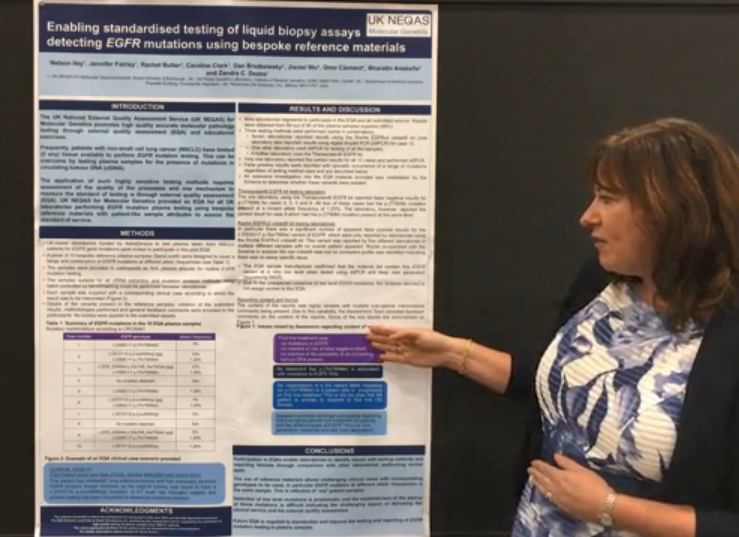
Video: See How an EQA Improves Clinical Genomics Testing with Affordable Custom Reference Standards
Category: NGS
Posted by
Ram Santhanam on Mar 6, 2019 12:00:00 AM
Next-generation sequencing (NGS) has revolutionized how assay developers, laboratories, and clinicians are diagnosing, treating, and monitoring disease. As NGS panels grow to include an increasing number of important biomarkers, so too must the reference standards used for development, validation, and routine QC.
0 Comments Click here to read/write comments
Ground Control to Major Tom: Use Controls and Keep Your Assay Running On
Category: qc management, NGS
Posted by
Lorn Davis on Feb 28, 2019 12:00:00 AM
Labs that demonstrate best practice clinical next-generation sequencing (NGS) quality management programs utilize positive run controls, designed for this purpose, to monitor assay analytical performance across each step of the clinical NGS workflow. Common parameters for assessing the analytical performance of a clinical NGS run include sensitivity (true positives), specificity (true negatives),
0 Comments Click here to read/write comments
The Full Authority Companion Diagnostic
Category: FDA, clinical genomics, NGS
Posted by
Yves Konigshofer, PhD on Feb 21, 2019 12:00:00 AM
It is very likely that on your last flight the turbofan engines were controlled by full authority digital engine controls – FADECs for short. FADECs have played a significant role in keeping airline ticket prices low (except during holidays) by continually adjusting engine parameters so that the engine operates with maximum fuel efficiency and within operational limits, allowing pilots to focus on other tasks.
0 Comments Click here to read/write comments
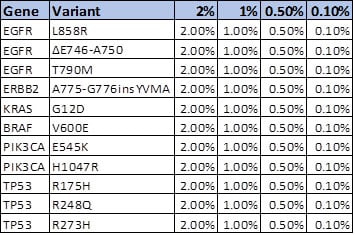
Customer Data: Use of Seraseq ctDNA Reference Samples to Validate Detection of Low Frequency Variants in a cfDNA-based NGS Lung Cancer Panel
Category: AMP, SeraSeq, cfDNA, Lung Cancer, ctDNA
Posted by
Omo Clement, PhD on Feb 14, 2019 12:00:00 AM
At the recently-concluded 2018 AMP Meeting, researchers at the New York Presbyterian Hospital (NYPH) and Weill Cornell Medical Center (WCMC) presented a poster1 on the validation of an Oncomine™ cell-free DNA Lung Assay using ctDNA NGS standards developed by SeraCare (Seraseq® ctDNA v2 Reference Materials),2
0 Comments Click here to read/write comments
NTRK Fusion Testing for New Therapies
Category: NTRK, cancer, RNA fusion
Posted by
Omo Clement, PhD on Feb 6, 2019 12:00:00 AM
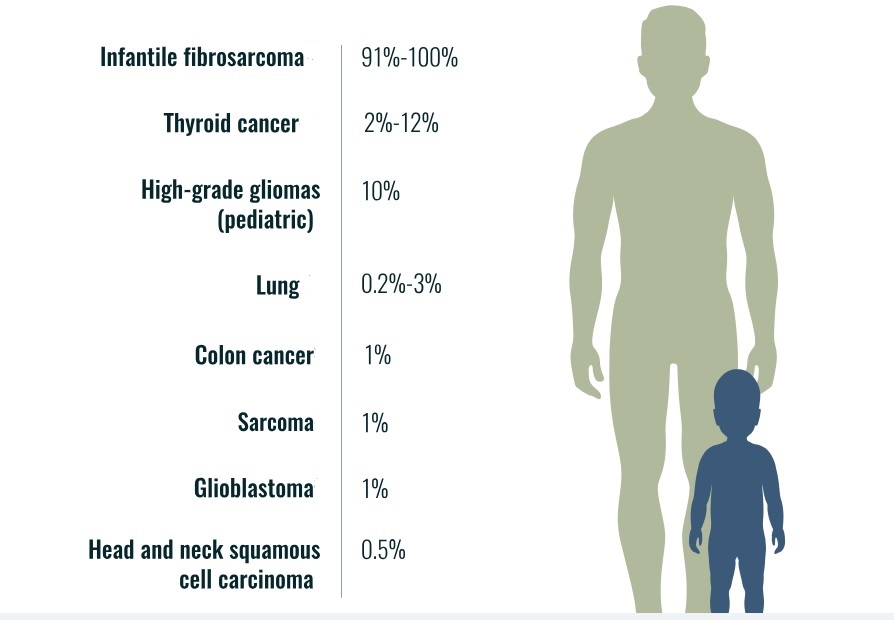
Neurotrophic tyrosine receptor kinases (NTRK) can become abnormally fused to other genes resulting in growth signals that can lead to cancer in many organs of the human body. TRK gene fusion-based cancers are rare but present in pediatric and adult cancers such as lung, thyroid, colon, etc. (see, e.g., Figure 1).
0 Comments Click here to read/write comments
Keys to Better Liquid Biopsy Assay Sensitivity – AMP Corporate Workshop Video
Category: AMP, liquid biopsy, NGS, ctDNA
Posted by
Sam Blier on Jan 24, 2019 12:00:00 AM
“So as everyone here is aware, I’m sure, detection of circulating tumor DNA is challenging. There’s very little of it, to start with.” Hardly a revolutionary statement by Tony Godfrey, PhD, (Associate Chair, Surgical Research and Associate Professor of Surgery, Boston University School of Medicine) but an important acknowledgement from a leading expert of the difficulty faced by laboratorians
0 Comments Click here to read/write comments
Workshop Video: Two Experts Take on Clinical Genomics QC and Standardization at AMP
Category: AMP, clinical genomics, NGS
Posted by
Sam Blier on Dec 18, 2018 12:00:00 AM
If you’ve attended the AMP Annual Meeting over the years or seen any of the headlines it generates, you know how next-generation sequencing-based assays are becoming indispensable diagnostic, prognostic, and predictive tools for a growing number of disease states. But just as important as the newest biomarker or latest chemistry – but seemingly less headline-worthy – are NGS quality control and standardization.
0 Comments Click here to read/write comments
Two Must-See Liquid Biopsy Poster Videos From AMP 2018
Category: AMP, SeraSeq, liquid biopsy, cfDNA, ctDNA
Posted by
Sam Blier on Dec 7, 2018 12:00:00 AM
Of the many fantastic posters presented at AMP’s Annual Meeting in San Antonio, two concerning NGS-based liquid biopsy assays stood out. Both presenters described how their organizations are working to reliably detect pathogenic variants at extremely low allele frequencies – efforts critical to the clinical adoption of NGS-based liquid biopsy assays.
0 Comments Click here to read/write comments