Choose your Article Focus | NGS | Molecular & Serology
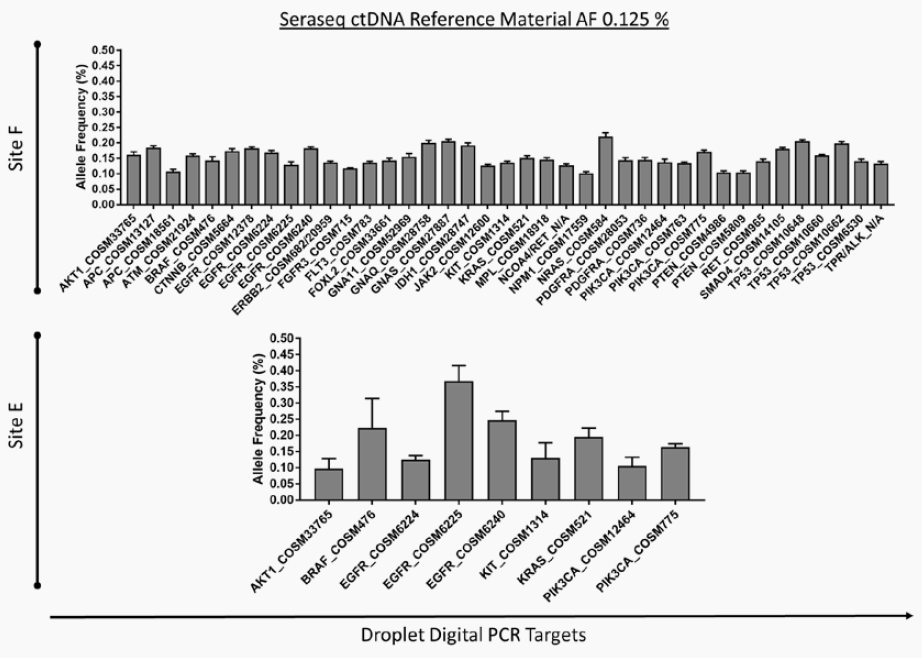
Building and Implementing Liquid Biopsy Assays with the Industry’s Most Patient-Like Reference Materials
Category: SeraSeq, liquid biopsy, NGS, reference materials
Posted by
Omo Clement, PhD on Oct 24, 2018 12:00:00 AM
SeraCare’s clinical genomics technologies are developed to address challenges faced across the spectrum of NGS assays. From early development of assays – either IVD assay manufacturers or clinical labs building their own LDTs - there is a scarcity of characterized, complex, difficult variants to ensure the assay can robustly detect all the critical genomic variants in a patient sample. Using our highly characterized, reproducible, and GMP-grade NGS standards, laboratories have a wide range of analytical and clinical validation tools to deeply characterize assay performance such as LOD, linearity, specificity, sensitivity, and reproducibility.
0 Comments Click here to read/write comments
To Realize the Potential of Liquid Biopsies, Focus on Higher Quality Research and Clinical Data Sets
Category: SeraSeq, liquid biopsy
Posted by
Dan Brudzewsky on Oct 15, 2018 12:00:00 AM
Liquid biopsy requires better standardization to realize all the new possibilities for studying metastasis, heterogenicity, treatment efficacy, and disease recurrence. Furthermore, it is critical for clinicians to have confidence in liquid biopsy data to diagnose and treat patients. This is only achievable when consistent and high-quality data is generated at research and all clinical centers. The Liquid Biopsies course at EMBL Advanced Training Centre provides a unique practical training in best practices and pitfalls on the complete liquid biopsy workflow, from sample preparation to data analysis. The course is targeted for clinical laboratory and research scientists interested in learning all aspects of liquid biopsy testing.
0 Comments Click here to read/write comments
Keep Calm and Standardize On
Category: qc management, QC Challenges, QC Management Software, NGS
Posted by
Peter Duncan on Oct 5, 2018 12:00:00 AM
There is that old adage that says the only thing that is constant is change. This is one of those universal truths we have all come to accept. Heck, even Dunkin' Donuts, widely credited as being the inventor of the word “Donut,” is dropping the word from their brand name. Blasphemy! But that is for another blog...
0 Comments Click here to read/write comments
As Immunotherapy Use Rises, Critical Gaps Remain in Harmonizing Tumor Mutational Burden Measurements
Posted by
Trevor Brown on Sep 19, 2018 12:00:00 AM
Recent clinical studies of immuno-oncology (I-O) checkpoint inhibitors have indicated that the tumor burden in a cancer patient’s genome may be predictive of positive response to I-O therapies such as Keytruda® and Opdivo®. The tumor mutational burden (TMB), that is, the number of mutations per megabase of sequenced tumor sample as determined by whole exome sequencing (WES), is currently the most promising biomarker for cancer patient selection and stratification in many clinical trials. Numerous clinical studies are underway to elucidate and validate the role of TMB in I-O treatment decision making and therapeutic response.
0 Comments Click here to read/write comments
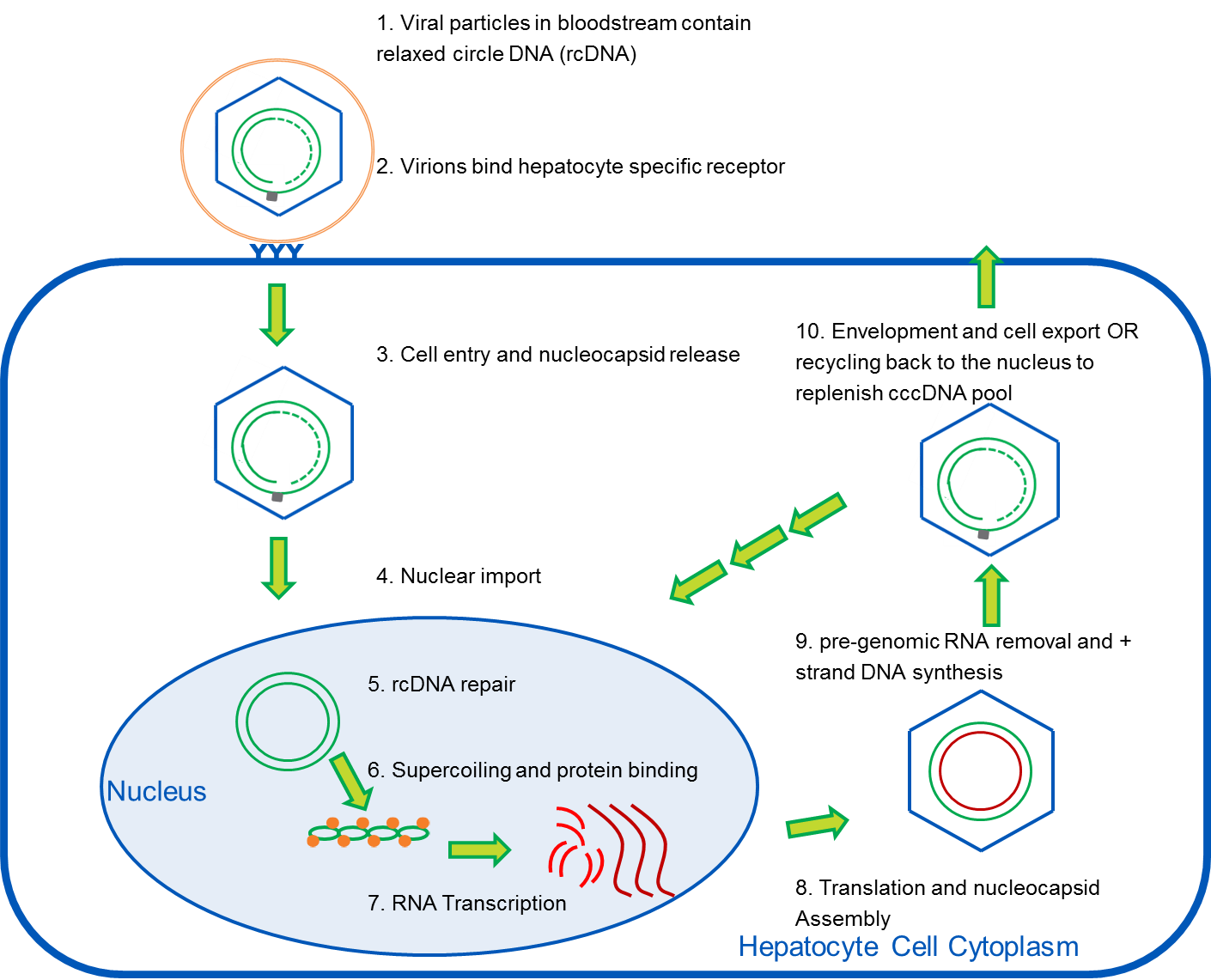
cccDNA and HBV: New Testing Methods May Allow for Earlier, Non-Invasive Detection of Hepatocellular Carcinoma
Posted by
Catherine Huang, PhD on Aug 23, 2018 12:00:00 AM
A recent study, published in the Journal of Molecular Diagnostics, describes a new, more sensitive Hepatitis B Virus (HBV) assay1. This study, led by Song-Mei Liu, MD, PhD, of the Center for Gene Diagnosis, Zhongnan Hospital of Wuhan University in China, is particularly exciting because the new assay can be used to diagnose hepatocellular carcinoma (HCC) at an earlier stage and to manage antiviral HBV treatments more effectively. It also highlights the way innovative molecular diagnostics can play a synergistic role with the development of new pharmaceutical therapeutics.
0 Comments Click here to read/write comments
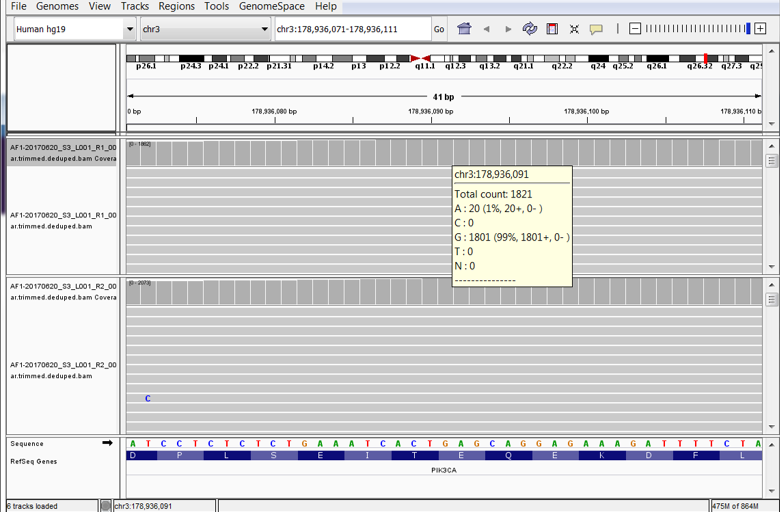
If I Call Out of Tune, Would Mutations Stand Up and Walk Out on Me?
Posted by
Matthew Butler on Aug 9, 2018 12:00:00 AM
One of several important steps in next-generation sequencing (NGS) is tuning the many options provided by mutation callers. Providing values for options configures the signal to noise ratio of the impending mutation calls. In theory, providing values that increase the stringency of mutation calls will reduce the number of false positive calls and thus enrich for true positives. In practice, increasing stringency can eliminate true positives.
0 Comments Click here to read/write comments
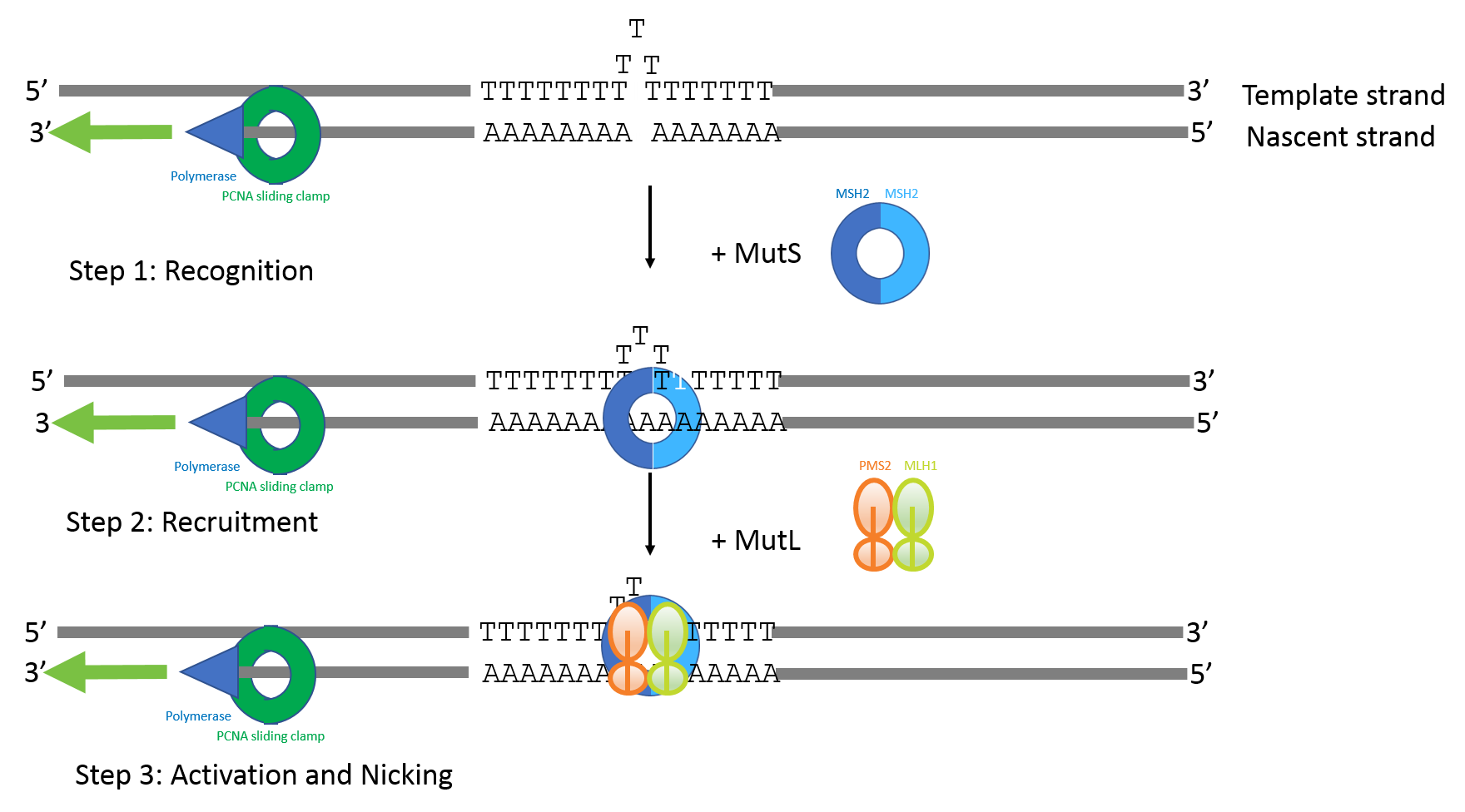
Microsatellite Instability Testing to Predict Immunotherapeutic Response: New Tools to Meet Testing Challenges
Category: NGS
Posted by
Catherine Huang, PhD on Jul 30, 2018 12:00:00 AM
Microsatellites are simple tandem repeats that are present at millions of sites in the human genome. Microsatellite Instability (MSI) is defined as a change of any length due to either insertion or deletion of repeating units in a microsatellite within a tumor compared with normal tissue.1 The molecular mechanism for the change in repeat length is slippage of nascent DNA strand with respect to the template strand during replication followed by failure to recognize the mismatch due to deficiency in mismatch repair genes.
0 Comments Click here to read/write comments
A First of its Kind Survey to Assess the QC Habits of Labs Running Clinical NGS Assays
Category: QC Challenges, NGS, QC Reporting
Posted by
Trevor Brown on Jul 12, 2018 12:00:00 AM
While good progress has been made of late with more clarity around FDA requirements, as well as organizations such as CAP and AMP providing more 'meat' in their guidance to clinical laboratories, there still remains a ways to go before this modality of testing is more standardized and uniform across the various laboratories offering the testing--be it via commercially available IVD kits or multiple different LDTs providing similar performance characteristics.
0 Comments Click here to read/write comments
Developing a Rock-Solid Lung Cancer Assay, Part 2
Category: NGS, cancer, Assay Development, Lung Cancer
Posted by
Yves Konigshofer, PhD on Jul 2, 2018 12:00:00 AM
In a recent post, we discussed key considerations for designing a robust next-generation sequencing (NGS)-based lung cancer assay. Putting those plans into action in the development phase brings forth a new set of challenges. Through our experience developing NGS reference materials and the relationships we’ve built with assay developers of all stripes, we’ve identified those important factors and ways to navigate them. But before you begin designing and optimizing your assay, you should become very familiar with binomial and Poisson distributions and their use because the outcome of many analytical steps can be modeled and explained with them.
0 Comments Click here to read/write comments
Ebola Outbreak 2018: Diagnostics Again are Essential to Minimize Spread and to Control Disease
Category: Accuplex, reference materials
Posted by
Catherine Huang, PhD on Jun 25, 2018 12:00:00 AM
On April 4th, 2018, a new outbreak of Ebola Virus Disease (EVD) occurred in Equateur Province in the Democratic Republic of the Congo. As of June 10th, there have been a total of 55 EVD cases and 28 deaths with a case fatality rate of 50.9%. Although the outbreak remains active, public health authorities have expressed cautious optimism because there have been no new cases in two of the three affected areas (Bikoro and Wangata zones) since May 17th, 2018 and the rate of new cases in the third affected zone (Iboko) has slowed.1
0 Comments Click here to read/write comments