Choose your Article Focus | NGS | Molecular & Serology
Multi-Lab Study of Fusion RNA Reference Standards for Targeted NGS
Category: NTRK, NGS, RNA fusion, reference materials, AACR
Posted by
Andrew Anfora, PhD on Jan 28, 2020 12:00:00 AM
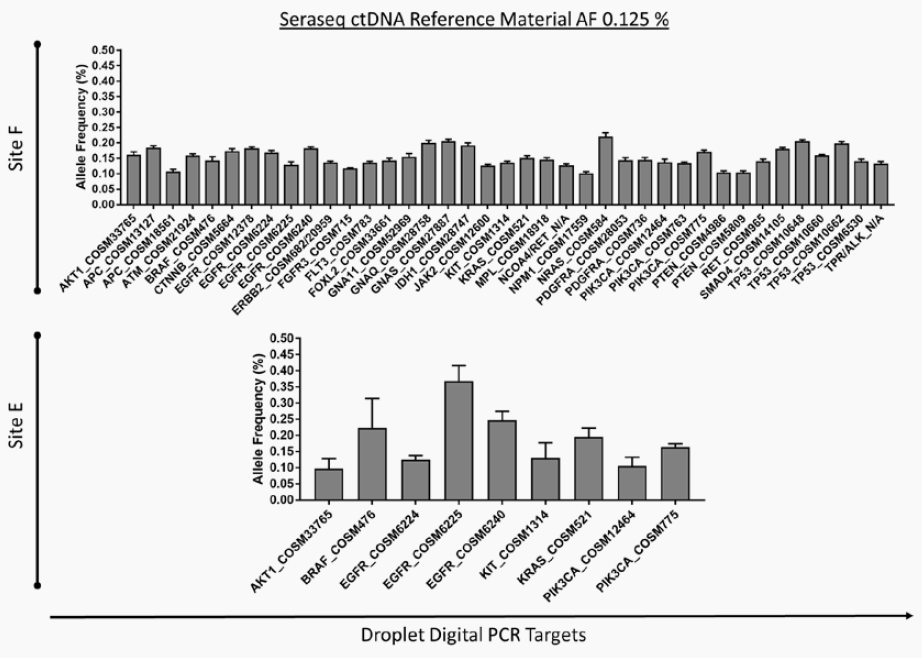
Sourcing assay validation samples as positive run controls or workflow controls in targeted NGS RNA fusion assays remains a challenge today. This is further exacerbated with clinical labs looking to provide validated NGS assays for patient stratification in a host of new drugs in clinical trials or newly approved targeting fusion genes, such as NTRK genes (Larotrectinib, Loxo/Bayer) and Entrectinib (Genentech/Roche) for rare cancers in adult and pediatric patients, and RET (Loxo/Lilly) for lung cancer. SeraCare produces several RNA fusion reference materials. This article describes the development and multi-laboratory evaluation of a pan-cancer multiplexed Fusion RNA reference standard for analysis of clinically relevant fusion genes in solid tumors. The evaluation was conducted at 5 different laboratories on different NGS platforms (amplicon- and hybridization capture-based) as well as at different RNA inputs within a platform. Results highlight the utility of this Fusion RNA reference material to support clinical NGS assays as positive controls in solid tumor cancer patient stratification for many of these fusion-based targeted therapies.
0 Comments Click here to read/write comments
What is Non-Invasive Prenatal Testing (NIPT)?
Category: New Reference Material, trisomy, Reproductive Health, NIPT, #Quality, reference materials
Posted by
SeraCare Team on Jan 20, 2020 12:00:00 AM
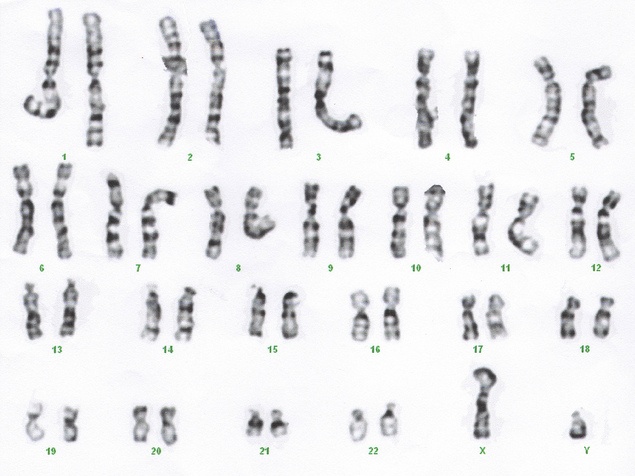
Fetal aneuploidy affects about 9 in 1,000 live births. The definition of aneuploidy is an abnormal number of chromosomes ; with 23 pairs of chromosomes in humans, 46 is the normal number, while aneuploidy individuals will have 45 or 47. In trisomy, there is one additional chromosome, typically chr21, 18 or 13 (it is not a coincidence that these are the smallest chromosomes in humans). Historically, the invasive methods amniocentesis and chorionic villus sampling (CVS) were used with risk to the pregnancy, with about a 1% chance of miscarriage due to the procedure. Non-invasive methods based upon ultrasound and serum biomarkers are useful screening tests, but were of limited reliability as they were indirect measures of chromosomal abnormalities1. Photograph courtesy of Flickr user Can H.
0 Comments Click here to read/write comments
Building and Implementing Liquid Biopsy Assays with the Industry’s Most Patient-Like Reference Materials
Category: SeraSeq, liquid biopsy, NGS, reference materials
Posted by
Omo Clement, PhD on Oct 24, 2018 12:00:00 AM
SeraCare’s clinical genomics technologies are developed to address challenges faced across the spectrum of NGS assays. From early development of assays – either IVD assay manufacturers or clinical labs building their own LDTs - there is a scarcity of characterized, complex, difficult variants to ensure the assay can robustly detect all the critical genomic variants in a patient sample. Using our highly characterized, reproducible, and GMP-grade NGS standards, laboratories have a wide range of analytical and clinical validation tools to deeply characterize assay performance such as LOD, linearity, specificity, sensitivity, and reproducibility.
0 Comments Click here to read/write comments
Ebola Outbreak 2018: Diagnostics Again are Essential to Minimize Spread and to Control Disease
Category: Accuplex, reference materials
Posted by
Catherine Huang, PhD on Jun 25, 2018 12:00:00 AM
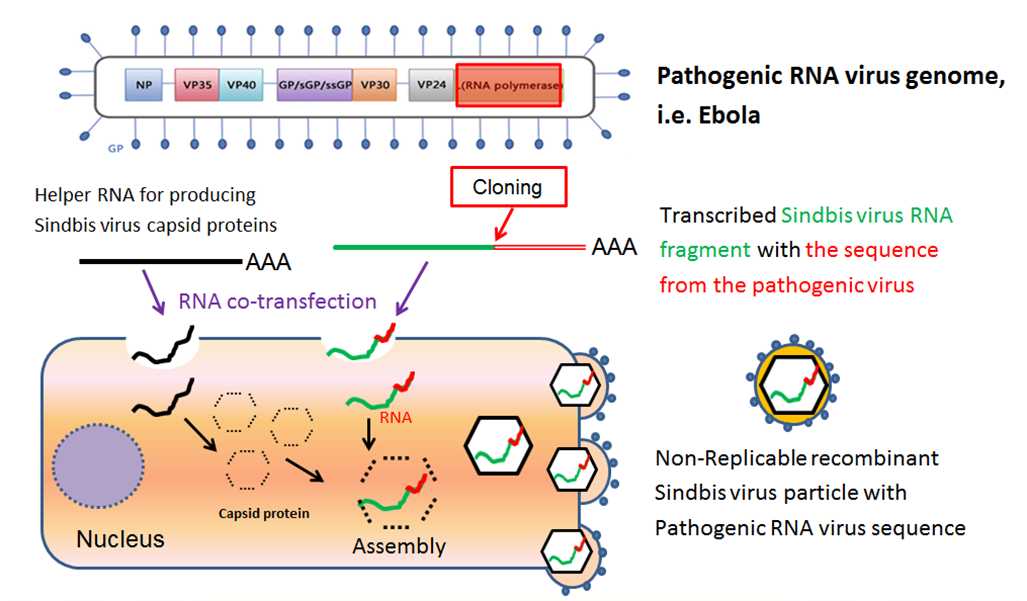
On April 4th, 2018, a new outbreak of Ebola Virus Disease (EVD) occurred in Equateur Province in the Democratic Republic of the Congo. As of June 10th, there have been a total of 55 EVD cases and 28 deaths with a case fatality rate of 50.9%. Although the outbreak remains active, public health authorities have expressed cautious optimism because there have been no new cases in two of the three affected areas (Bikoro and Wangata zones) since May 17th, 2018 and the rate of new cases in the third affected zone (Iboko) has slowed.1
0 Comments Click here to read/write comments
Developing a Rock-Solid Lung Cancer Assay
Category: NGS, cancer, Lung Cancer, reference materials
Posted by
Yves Konigshofer, PhD on Mar 15, 2018 12:00:00 AM
Next-generation sequencing (NGS) allows deeper insights than ever before into the human genome and a host of diseases and conditions. So it makes sense that there is a worldwide movement to employ NGS in a growing number of applications. But as the saying goes, with great power comes great responsibility.
0 Comments Click here to read/write comments
CNVs and Tumor Profiling: New CNV Materials for Breast, Lung, and Brain Cancer
Category: NGS, cancer, reference materials
Posted by
Dana Ruminski Lowe, Ph.D. on Feb 14, 2018 12:00:00 AM
Simply described, copy number variations (CNVs) are DNA segments present at a variable copy number in comparison to a normal genome. It was originally thought that a CNV consisted of a region of greater than 1 kilobases, however advances in technology have allowed for identification of CNVs as small as 50 basepairs1.
0 Comments Click here to read/write comments
With so many options, how do you select the best NGS cancer assay?
Category: NGS, cancer, RNA fusion, reference materials
Posted by
Catherine Huang, PhD on Jan 11, 2018 12:00:00 AM
Clinical labs must constantly evolve their test offerings in order to support the most recent advances in clinical care. For next-generation sequencing (NGS) tumor profiling assays, there are often multiple commercially available kits with similar claims for gene content and sensitivity, as well as customized solutions. How can you quickly perform an effective evaluation of available assay systems to make a data-driven choice?
0 Comments Click here to read/write comments
AMP Reference Material Forum: Themes and Highlights
Category: AMP, clinical genomics, reference materials
Posted by
Catherine Huang, PhD on Dec 22, 2017 12:00:00 AM
On November 14, 2017, AMP hosted a forum to discuss genetic testing reference material availability and needs. The forum attracted attendees including EQA providers, developers in industry and government, as well as scientists from clinical laboratories. Topics for discussion included reference material use and needs for assay validation, quality control, and proficiency testing. Throughout the talks, a few themes emerged and were discussed by multiple speakers.
0 Comments Click here to read/write comments
The First Comprehensive Myeloid Cancer Reference Materials for NGS Assays
Category: myeloid cancer reference materials, NGS, reference materials
Posted by
Ram Santhanam on Nov 8, 2017 12:00:00 AM
Myeloid cancers are “liquid” tumors that arise from the blood and bone marrow. These diseases have undergone greater study and characterization than perhaps any other type of cancer, largely due to the ease of accessing these cancer cells via a blood draw rather than a tissue biopsy, as for solid tumors. There are many different types and subtypes of these malignancies that are known to be caused by mutations in genes that encode proteins involved in cell signaling, transcription, epigenetic regulation, and splicing1. Before next-generation sequencing became available in the hematology/oncology clinic, high-resolution genetic analysis of myeloid cancers relied primarily upon site-specific methods such as Fluorescence in Situ Hybridization (FISH) and PCR-based assays. And, while other methods such as karyotyping and array comparative genomic hybridization are indeed able to survey large genomic rearrangements and copy number changes across the entire genome, these methods lack the resolution required for detection of many mutations that are important for myeloid cancers.
0 Comments Click here to read/write comments
Is Your NGS-Based Assay on the Right TRK?
Category: qc management, QC Management Software, NGS, RNA fusion, reference materials
Posted by
Trevor Brown on Oct 9, 2017 12:00:00 AM
Despite the absence of clear guidelines or firmly established best practices, next-generation sequencing (NGS) assays are becoming the method of choice for gene fusion detection. This is significant because, although some of the cancers that contain fusion RNAs are rare, they’re now treatable thanks to new targeted therapies. If your assay can detect fusion RNAs, it can help profile tumors for important diagnostic, prognostic, and therapeutic targets, which can lead to improved patient outcomes. The old FISH method limited you to one type of fusion variant at a time; it was effective, but also slow and cumbersome. With the latest NGS techniques, detecting fusion RNAs is more efficient than ever. It’s more sensitive and can detect multiple fusions in the same assay. Nevertheless, it’s still challenging because of the complex workflows and the need to rigorously ensure performance across all fusion variants. From extraction, to library prep, to sequencing, to the bioinformatics pipeline, there are countless points where something could go wrong.
0 Comments Click here to read/write comments