Choose your Article Focus | NGS | Molecular & Serology
Accelerating Liquid Biopsy Assay Development
Category: liquid biopsy, Assay Development, ctDNA, reference materials
Posted by
Sam Blier on Sep 8, 2017 12:00:00 AM
At the 2017 Next Generation Dx Summit in Washington, DC, our CSO, Russell Garlick, PhD, presented a workshop on accelerating liquid biopsy assay development. He has worked closely with a variety of groups in the liquid biopsy space that are developing and validating circulating tumor DNA (ctDNA) assays. He highlighted some common challenges facing the field, and explained how SeraCare has been using these collaborations to develop QC tools specifically for ensuring the robustness of these cutting-edge tests.
0 Comments Click here to read/write comments
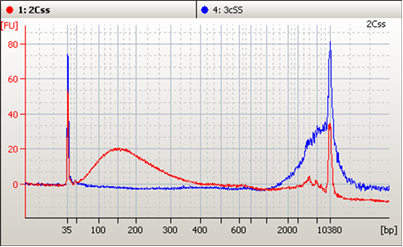
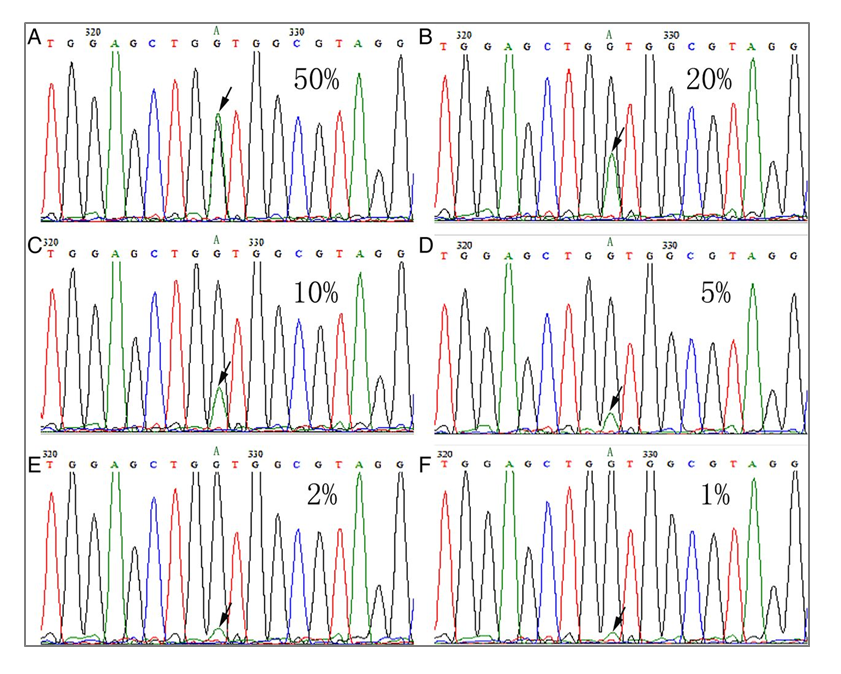
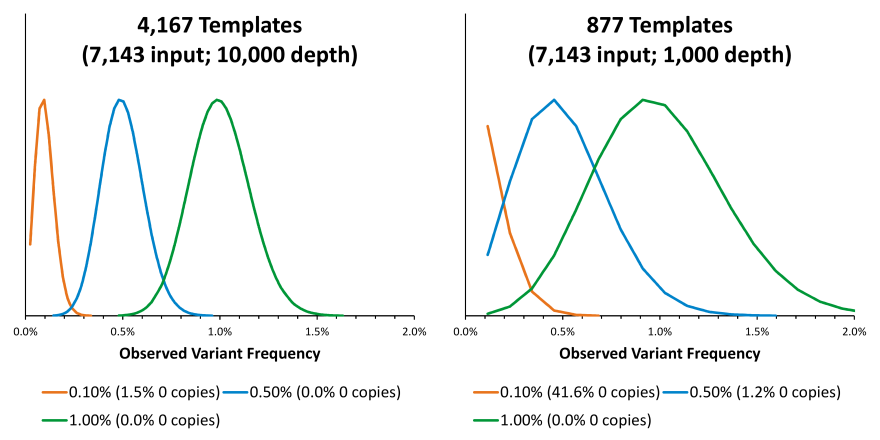
Three liquid biopsy problems solved by the most patient-like ctDNA reference materials
Category: cfDNA, ctDNA, reference materials
Posted by
Dale Yuzuki on Jul 24, 2017 12:00:00 AM
Clinical genomics laboratories are increasingly looking to liquid biopsy cancer assays to complement their current solid tumor assays. Compared to their solid tumor assay counterparts, circulating tumor DNA (ctDNA) assays offer a different set of challenges to consider for clinical labs. One of the most important of which, is to develop a set of reagents that are appropriately validated to determine the critical performance of the assay across many parameters. The ctDNA targets of liquid biopsy assays are typically at much lower allelic frequencies and require a robust and reproducibly designed assay to consistently detect these important variants.
0 Comments Click here to read/write comments
Dr. Andrea Ferreira-Gonzalez on the Seven Benefits of Clinical Genomics Universal Standardization
Category: clinical genomics, laboratory training, NGS, reference materials
Posted by
Dale Yuzuki on Jun 22, 2017 12:00:00 AM
As a 25-year veteran of clinical molecular diagnostics, Dr. Andrea Ferreira-Gonzalez has seen many changes in genetic technologies used in the testing laboratory. With the advent of personalized medicine and using multi-gene NGS panels as a laboratory-developed test, Dr. Ferreira-Gonzalez and other experts have agreed to lend their expertise to the design of SeraCare’s reference materials. She and other groups have participated in an interlaboratory test of standardized reference materials for detecting cancer somatic mutations, with results that will be published in the coming months.
0 Comments Click here to read/write comments
Real-World Needs for Genetic Testing Reference Materials
Category: NGS, reference materials
Posted by
Matt Ryder on Jun 1, 2017 12:00:00 AM
“Happy families are all alike; every unhappy family is unhappy in its own way.” I have always thought the opening of Anna Karenina applies for many things beyond familial harmony (or lack thereof). Certainly, in the world of molecular genetic diagnostics, conclusive results are usually obtained for most patients; however, there are times when a final result is more elusive than conclusive. When this occurs, it may seem as though no two challenges are ever the same. The following are real examples – presented in general terms for patient and institutional confidentiality – of difficult, unanticipated, and even bizarre cases I encountered during my time in clinical testing for predisposition to hereditary disease. Each of these situations required extraordinary effort, dedicated time, and additional resources for resolution. At the end of each day, satisfaction came from knowing that another problem solved was another patient helped in making life-altering medical management decisions.
0 Comments Click here to read/write comments
Clinical Laboratories: You Are Not Alone. (Part II)
Category: QC Challenges, NGS, ctDNA, reference materials
Posted by
Matt Ryder on Dec 7, 2016 12:00:00 AM
Previously, we wrote about some of the Quality Control challenges that clinical laboratories performing Next Generation Sequencing (NGS) face towards ensuring their assays are safe and effective for guiding medical management decisions. Reliable access to high quality reference materials is necessary to help overcome these challenges; however, it is not sufficient. Insights that reference materials provide into the health of an NGS assay are only as good as laboratories’ ability to use their QC data effectively. With limited time and resources to collect, organize, access, and analyze QC metrics, laboratories may frequently rely on reference materials as binary indicators of Pass/Fail: As long as the expected endpoint results are obtained, an assay is considered to be performing well. The drawback of this approach is that it is reactive, rather than proactive: A sufficient number of failures must occur within a given timeframe before a troubleshooting investigation is performed. By the time a problem is recognized, resources have been wasted and turnaround times (TAT) delayed; in some cases, fidelity of patient results may even have been put at risk. Additional time and costs are then incurred as the investigation proceeds. Specimen analysis by NGS yields a wealth of information in addition to endpoint variant calls that is indicative of assay performance. Data such as nucleic acid quantity and quality at different steps throughout the workflow (PDF) and sequencing library characteristics are generated every time a reference material is tested. However, these data must be carefully tracked and trended to allow use as highly informative QC parameters. For clinical laboratories whose primary focus is on patient testing and reporting, granular QC metrics may not be captured and reviewed as part of routine test monitoring.
0 Comments Click here to read/write comments
Clinical Laboratories: You Are Not Alone.
Category: NGS, reference materials
Posted by
Matt Ryder on Nov 8, 2016 12:00:00 AM
Since the introduction of the GS20 in 2005 by 454 Life Sciences, Next Generation Sequencing (NGS) has found many applications in clinical diagnostics. As a result of this transition from the long-held gold standard, Sanger sequencing, the primary challenge for clinical laboratories has shifted from data acquisition to ensuring these tests are safe and effective for guiding medical management decisions. Many laboratories struggle to gain a thorough understanding of the analytic performance characteristics of their NGS tests. The difficulty arises from the fact that these assays are comprised of highly complex, fragmented workflows, and have many different intended uses. However, across the various practices currently used for NGS assay development, validation, and performance monitoring, there is a common goal: results must be as accurate, precise, and consistent as possible.
0 Comments Click here to read/write comments
Reference Materials for Your Unique Reproducibility Needs
Category: FDA, SeraSeq, clinical genomics, NGS, reference materials
Posted by
Matt Ryder on Oct 12, 2016 12:00:00 AM
If you took a university introductory statistics course, you may have learned the distinction between accuracy and precision. It may likely have been presented with an archery analogy, where ‘Accurate’ was represented by arrows loosely clustered around the target’s bull’s-eye, ‘Precise’ was shown as a tight grouping displaced from the center, and ‘Accurate and Precise’ was depicted as what every archer aims for, a tight grouping directly at the bull’s-eye. Suddenly, words that are used interchangeably in everyday conversation took on dramatically different meanings.
0 Comments Click here to read/write comments
FDA-AACR Liquid Biopsies in Oncology Drug and Device Workshop
Category: FDA, clinical genomics, NGS, cancer, LDT, ctDNA, reference materials
Posted by
Yves Konigshofer, PhD on Aug 8, 2016 12:00:00 AM
The presentations during the FDA-AACR Liquid Biopsies in Oncology Drug and Device Development Workshop on July 19, 2016 included several important pieces of information that will likely guide the development of assays and their review by the FDA.
0 Comments Click here to read/write comments
IVD Guidance for NGS Manufacturers
Category: FDA, clinical genomics, NGS, LDT, reference materials
Posted by
Russell Garlick, PhD on Jul 14, 2016 12:00:00 AM
After 17 months of deliberations since its first open meeting February 20, 2015 on NGS IVD assay oversight, the U.S. Food and Drug Administration (FDA) issued DRAFT guidance for Stakeholders and FDA staff. The document “Use of Standards in FDA Regulatory Oversight of Next Generation Sequencing (NGS)-Based In Vitro Diagnostics (IVDs) Used for Diagnosing Germline Diseases” was published online on July 6, 2016. This document (PDF located here) is for analytical validity and not for clinical validation.
0 Comments Click here to read/write comments
Highlights from the 2016 Molecular Medicine Tri-Conference
Category: clinical genomics, cancer, Inherited Disease, ctDNA, reference materials
Posted by
Dale Yuzuki on Mar 22, 2016 12:00:00 AM
We are living through a time of rapid change in the clinical genetics laboratory, though at times it may appear that change doesn’t occur fast enough given the challenges within the existing healthcare system. At the recent Molecular Medicine Tri-Conference held in San Francisco March 7 through 11 2016, here are a few summary highlights of the conference.
0 Comments Click here to read/write comments