Choose your Article Focus | NGS | Molecular & Serology
Developing a Rock-Solid Lung Cancer Assay, Part 2
Category: NGS, cancer, Assay Development, Lung Cancer
Posted by
Yves Konigshofer, PhD on Jul 2, 2018 12:00:00 AM
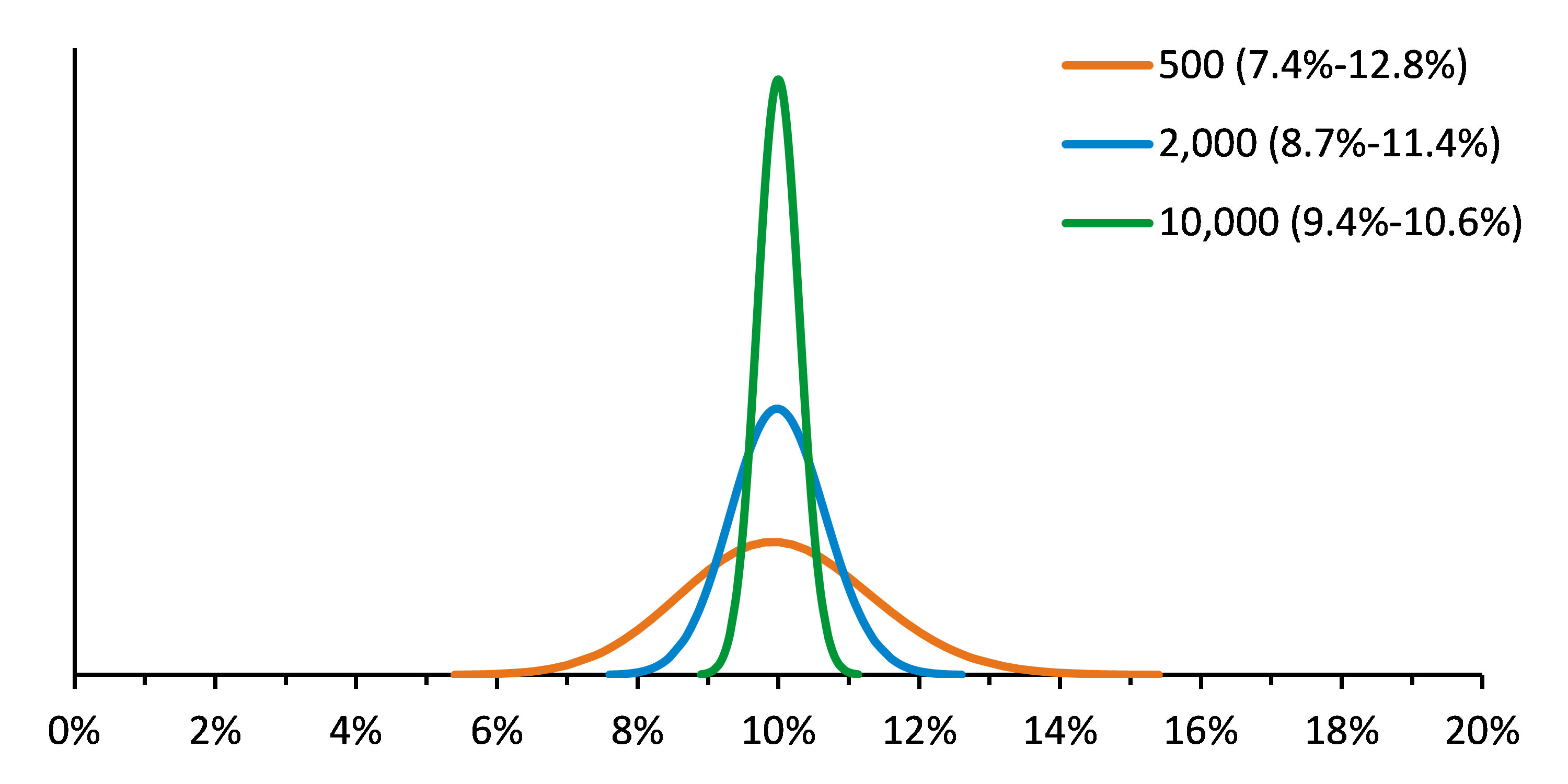
In a recent post, we discussed key considerations for designing a robust next-generation sequencing (NGS)-based lung cancer assay. Putting those plans into action in the development phase brings forth a new set of challenges. Through our experience developing NGS reference materials and the relationships we’ve built with assay developers of all stripes, we’ve identified those important factors and ways to navigate them. But before you begin designing and optimizing your assay, you should become very familiar with binomial and Poisson distributions and their use because the outcome of many analytical steps can be modeled and explained with them.
0 Comments Click here to read/write comments
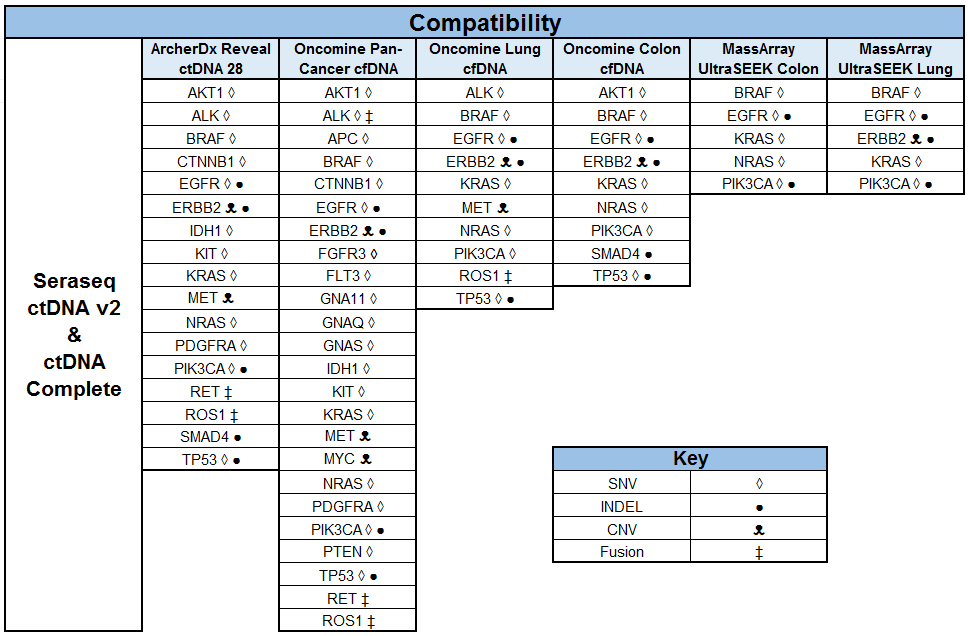
How A New Generation of ctDNA Reference Standards Are Enabling the Promise of Precision Medicine
Category: SeraSeq, liquid biopsy, NGS, cancer, ctDNA
Posted by
Omo Clement, PhD on Jun 14, 2018 12:00:00 AM
An important goal in cancer disease management is early detection. When detected early, disease progression can be significantly mitigated with a plethora of options (targeted therapy, chemotherapy, surgery, etc.) available to medical practitioners, to afford progression free survival and a higher quality of life. A great promise of liquid biopsies is the possibility of early detection of cancer long before clear evidence of lesions and tumor growth observable by imaging or other techniques.1 As proxy for solid tissue biopsies, plasma-based liquid biopsy application is rapidly gaining traction in cancer disease diagnosis, progression, monitoring, and in predicting resistance to treatment options.2
0 Comments Click here to read/write comments
FAQ: What to do when your NGS assay fails to detect a variant contained in Seraseq Reference Materials?
Posted by
Catherine Huang, PhD on Jun 11, 2018 12:00:00 AM
Highly multiplexed reference materials are particularly valuable when developing and optimizing new NGS assays because they allow you to evaluate the performance of your assay across a large number of variants including different variant types (SNVs, indels, homopolymeric variants, etc.) and contexts. However, it can be frustrating when a variant in the reference material is not detected, or not detected at the expected variant allele frequency. Troubleshooting such issues can give new insight into the performance of the assay. Here we share some stories from Seraseq™ users where the lack of detection of one or more variants at the expected levels helped them improve their assay or set more appropriate QC thresholds.
0 Comments Click here to read/write comments
Developing a Rock-Solid Lung Cancer Assay
Category: NGS, cancer, Lung Cancer, reference materials
Posted by
Yves Konigshofer, PhD on Mar 15, 2018 12:00:00 AM
Next-generation sequencing (NGS) allows deeper insights than ever before into the human genome and a host of diseases and conditions. So it makes sense that there is a worldwide movement to employ NGS in a growing number of applications. But as the saying goes, with great power comes great responsibility.
0 Comments Click here to read/write comments
An Efficient and Ultrasensitive NGS Solution for Profiling ctDNA [Poster Talk Video]
Category: liquid biopsy, NGS, ctDNA
Posted by
Trevor Brown on Mar 5, 2018 12:00:00 AM
SeraCare Customer Poster Talk Video with Data Presented by Asuragen Next-generation sequencing (NGS) of liquid biopsies offers a minimally invasive alternative to solid tissue biopsies and a more holistic profile of intra- and inter-tumoral heterogeneity for therapy selection and disease monitoring. Watch the video and download this free poster to learn:
0 Comments Click here to read/write comments
An Efficient and Ultrasensitive NGS Solution for Profiling ctDNA [Poster Talk Video]
Category: liquid biopsy, NGS, ctDNA
Posted by
Trevor Brown on Feb 28, 2018 12:00:00 AM
SeraCare Customer Poster Talk Video with Data Presented by Asuragen Next-generation sequencing (NGS) of liquid biopsies offers a minimally invasive alternative to solid tissue biopsies and a more holistic profile of intra- and inter-tumoral heterogeneity for therapy selection and disease monitoring. Watch the video and download this free poster to learn: How reference materials that commute to the target sample type can help to optimize ctDNA profiling technology Why the Seraseq ctDNA v2 Reference Material most closely resembles native ctDNA in amplifiablity and molecular diversity How highly patient-like reference materials allowed confident quantification of trace levels of ctDNA
0 Comments Click here to read/write comments
CNVs and Tumor Profiling: New CNV Materials for Breast, Lung, and Brain Cancer
Category: NGS, cancer, reference materials
Posted by
Dana Ruminski Lowe, Ph.D. on Feb 14, 2018 12:00:00 AM
Simply described, copy number variations (CNVs) are DNA segments present at a variable copy number in comparison to a normal genome. It was originally thought that a CNV consisted of a region of greater than 1 kilobases, however advances in technology have allowed for identification of CNVs as small as 50 basepairs1.
0 Comments Click here to read/write comments
With so many options, how do you select the best NGS cancer assay?
Category: NGS, cancer, RNA fusion, reference materials
Posted by
Catherine Huang, PhD on Jan 11, 2018 12:00:00 AM
Clinical labs must constantly evolve their test offerings in order to support the most recent advances in clinical care. For next-generation sequencing (NGS) tumor profiling assays, there are often multiple commercially available kits with similar claims for gene content and sensitivity, as well as customized solutions. How can you quickly perform an effective evaluation of available assay systems to make a data-driven choice?
0 Comments Click here to read/write comments
How am I going to test my assay? Should I use patient samples or biosynthetic materials?
Category: NGS, Assay Development
Posted by
Dan Brudzewsky on Jan 4, 2018 12:00:00 AM
Assay development and optimization for clinical genetics is increasingly challenging. In an era of clinical genomics, new technologies and clinical utilities constantly call for newer and better performing assays. Having access to an abundant supply of relevant and reliable test material is critical for quick assay development and well-documented assay performance.
0 Comments Click here to read/write comments
As to Myeloid Diseases, Make a Habit of Three Things
Category: myeloid cancer reference materials, NGS
Posted by
Ram Santhanam on Dec 13, 2017 12:00:00 AM
Previously, we wrote about the unique capabilities that next-generation sequencing (NGS) offers the oncology clinic. NGS could mark the beginning of a shift away from “single-site” technologies such as FISH and PCR-based testing, in favor of comprehensive screening across many different targets at once.
0 Comments Click here to read/write comments