Choose your Article Focus | NGS | Molecular & Serology
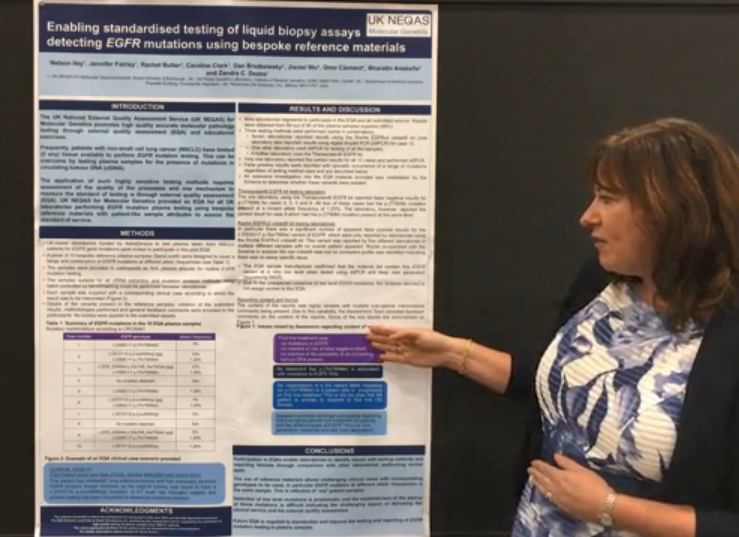
Video: See How an EQA Improves Clinical Genomics Testing with Affordable Custom Reference Standards
Category: NGS
Posted by
Ram Santhanam on Mar 6, 2019 12:00:00 AM
Next-generation sequencing (NGS) has revolutionized how assay developers, laboratories, and clinicians are diagnosing, treating, and monitoring disease. As NGS panels grow to include an increasing number of important biomarkers, so too must the reference standards used for development, validation, and routine QC.
0 Comments Click here to read/write comments
Ground Control to Major Tom: Use Controls and Keep Your Assay Running On
Category: qc management, NGS
Posted by
Lorn Davis on Feb 28, 2019 12:00:00 AM
Labs that demonstrate best practice clinical next-generation sequencing (NGS) quality management programs utilize positive run controls, designed for this purpose, to monitor assay analytical performance across each step of the clinical NGS workflow. Common parameters for assessing the analytical performance of a clinical NGS run include sensitivity (true positives), specificity (true negatives),
0 Comments Click here to read/write comments
The Full Authority Companion Diagnostic
Category: FDA, clinical genomics, NGS
Posted by
Yves Konigshofer, PhD on Feb 21, 2019 12:00:00 AM
It is very likely that on your last flight the turbofan engines were controlled by full authority digital engine controls – FADECs for short. FADECs have played a significant role in keeping airline ticket prices low (except during holidays) by continually adjusting engine parameters so that the engine operates with maximum fuel efficiency and within operational limits, allowing pilots to focus on other tasks.
0 Comments Click here to read/write comments
Keys to Better Liquid Biopsy Assay Sensitivity – AMP Corporate Workshop Video
Category: AMP, liquid biopsy, NGS, ctDNA
Posted by
Sam Blier on Jan 24, 2019 12:00:00 AM
“So as everyone here is aware, I’m sure, detection of circulating tumor DNA is challenging. There’s very little of it, to start with.” Hardly a revolutionary statement by Tony Godfrey, PhD, (Associate Chair, Surgical Research and Associate Professor of Surgery, Boston University School of Medicine) but an important acknowledgement from a leading expert of the difficulty faced by laboratorians
0 Comments Click here to read/write comments
Workshop Video: Two Experts Take on Clinical Genomics QC and Standardization at AMP
Category: AMP, clinical genomics, NGS
Posted by
Sam Blier on Dec 18, 2018 12:00:00 AM
If you’ve attended the AMP Annual Meeting over the years or seen any of the headlines it generates, you know how next-generation sequencing-based assays are becoming indispensable diagnostic, prognostic, and predictive tools for a growing number of disease states. But just as important as the newest biomarker or latest chemistry – but seemingly less headline-worthy – are NGS quality control and standardization.
0 Comments Click here to read/write comments
Building and Implementing Liquid Biopsy Assays with the Industry’s Most Patient-Like Reference Materials
Category: SeraSeq, liquid biopsy, NGS, reference materials
Posted by
Omo Clement, PhD on Oct 24, 2018 12:00:00 AM
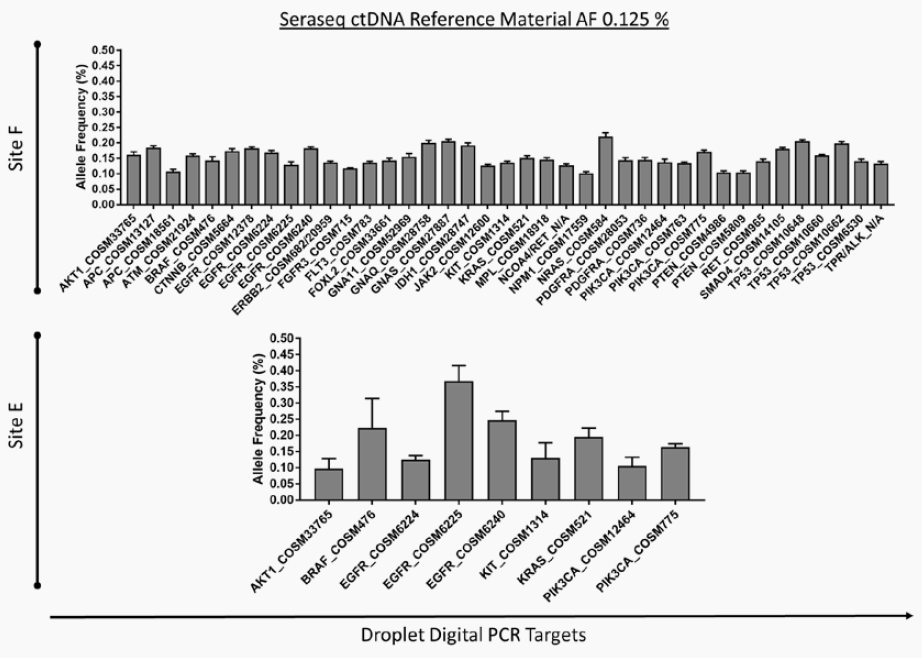
SeraCare’s clinical genomics technologies are developed to address challenges faced across the spectrum of NGS assays. From early development of assays – either IVD assay manufacturers or clinical labs building their own LDTs - there is a scarcity of characterized, complex, difficult variants to ensure the assay can robustly detect all the critical genomic variants in a patient sample. Using our highly characterized, reproducible, and GMP-grade NGS standards, laboratories have a wide range of analytical and clinical validation tools to deeply characterize assay performance such as LOD, linearity, specificity, sensitivity, and reproducibility.
0 Comments Click here to read/write comments
Keep Calm and Standardize On
Category: qc management, QC Challenges, QC Management Software, NGS
Posted by
Peter Duncan on Oct 5, 2018 12:00:00 AM
There is that old adage that says the only thing that is constant is change. This is one of those universal truths we have all come to accept. Heck, even Dunkin' Donuts, widely credited as being the inventor of the word “Donut,” is dropping the word from their brand name. Blasphemy! But that is for another blog...
0 Comments Click here to read/write comments
If I Call Out of Tune, Would Mutations Stand Up and Walk Out on Me?
Posted by
Matthew Butler on Aug 9, 2018 12:00:00 AM
One of several important steps in next-generation sequencing (NGS) is tuning the many options provided by mutation callers. Providing values for options configures the signal to noise ratio of the impending mutation calls. In theory, providing values that increase the stringency of mutation calls will reduce the number of false positive calls and thus enrich for true positives. In practice, increasing stringency can eliminate true positives.
0 Comments Click here to read/write comments
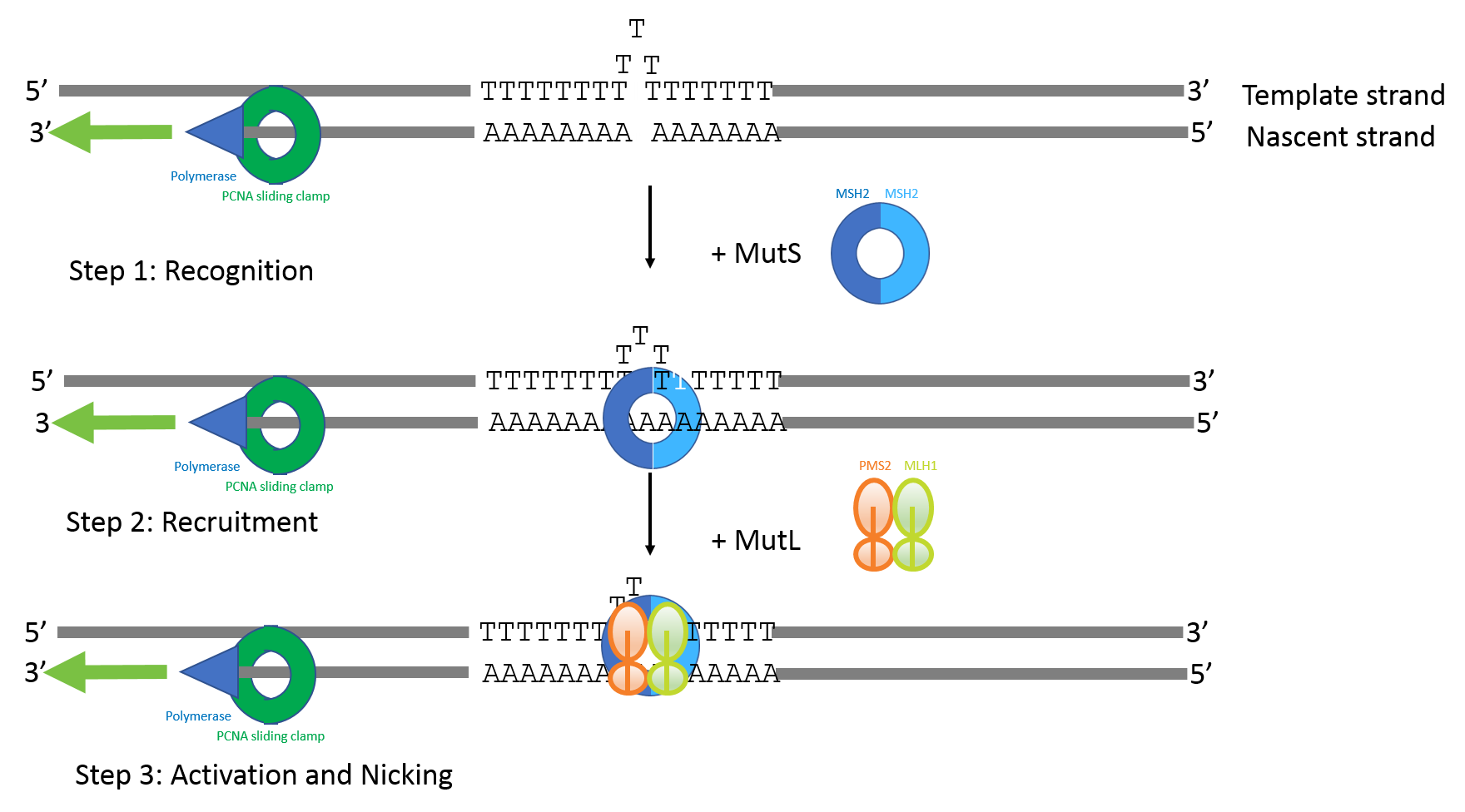
Microsatellite Instability Testing to Predict Immunotherapeutic Response: New Tools to Meet Testing Challenges
Category: NGS
Posted by
Catherine Huang, PhD on Jul 30, 2018 12:00:00 AM
Microsatellites are simple tandem repeats that are present at millions of sites in the human genome. Microsatellite Instability (MSI) is defined as a change of any length due to either insertion or deletion of repeating units in a microsatellite within a tumor compared with normal tissue.1 The molecular mechanism for the change in repeat length is slippage of nascent DNA strand with respect to the template strand during replication followed by failure to recognize the mismatch due to deficiency in mismatch repair genes.
0 Comments Click here to read/write comments
A First of its Kind Survey to Assess the QC Habits of Labs Running Clinical NGS Assays
Category: QC Challenges, NGS, QC Reporting
Posted by
Trevor Brown on Jul 12, 2018 12:00:00 AM
While good progress has been made of late with more clarity around FDA requirements, as well as organizations such as CAP and AMP providing more 'meat' in their guidance to clinical laboratories, there still remains a ways to go before this modality of testing is more standardized and uniform across the various laboratories offering the testing--be it via commercially available IVD kits or multiple different LDTs providing similar performance characteristics.
0 Comments Click here to read/write comments